Research from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) published in the Journal of Clinical Oncology demonstrates that hematopoietic cell transplantation (HCT) provides a significant survival benefit for older patients with myelodysplastic syndromes (MDS).
BMT CTN 1102 was a multi-center biologic assignment trial comparing reduced intensity allogeneic HCT to hypomethylating therapy or best supportive care in patients aged 50-75 with advanced MDS.
Download the below study summary with journal citations.
Research Highlights for Community Hematologists/Oncologists
To improve patient outcomes, there must be a shift toward earlier and more frequent referral of patients with MDS to transplant centers for consultation.
Recent advances in the treatment of MDS have improved overall survival (OS) and quality of life (QOL) for patients, while simultaneously reducing transfusion burden.
Although allogeneic HCT is the only potentially curative therapy for patients with MDS, it is not utilized extensively due to multiple barriers, ranging from referring physician perceptions about age criteria or donor options to insurance coverage concerns.
The large population of older patients that could benefit from HCT with the introduction of reduced intensity conditioning (RIC) regimens and alternative donor sources has therefore been overlooked.
The BMT CTN conducted a robust multi-center biologic assignment trial in older patients with high-risk MDS.
The researchers sought to better define the benefit of RIC HCT over non-HCT therapy.
Results support prompt and early HCT referral for patients with higher-risk MDS, as well as expanded insurance coverage for HCT procedures.
Patients with an allogeneic donor identified within 90 days of initiating a search who underwent HCT demonstrated 3-year OS of 47.9% compared with 26.6% for patients without a donor who received DNA hypomethylating agents (HMA) or best supportive care (p=0.0001). Preliminary analyses of QOL showed no clinically significant difference.
BMT CTN 1102 study summary
Objectives and rationale
The relative benefits of HCT over non-HCT therapy for older patients with MDS are not well defined, despite the need and frequency of resistance to HMA therapy.
The primary objective of this study was to compare RIC HCT to non-transplant therapies for higher-risk MDS based on donor availability between two study arms using intent-to-treat analysis with a primary outcome of 3-year OS.
The secondary objectives were to compare leukemia free survival (LFS) at 3 years from enrollment, QOL measures between arms, and cost-effectiveness between treatment arms.
Design and methods
The study was a multi-center, prospective biologic assignment trial in subjects aged 50-75 years with higher-risk de novo MDS (IPSS Intermediate-2 [Int2] or High) who were candidates for RIC allogeneic HCT. Biological assignment to the Donor or No Donor arm was based on human leukocyte antigen (HLA) typing and availability of an 8/8 HLA-matched family member or unrelated donor. Donor arm patients were expected to undergo RIC HCT within 6 months of enrollment.
The primary analysis compared OS between arms using adjusted survival estimates to account for potential bias from biologic assignment. The sample size was selected to provide at least 80% power to detect a difference of 15% in 3-year OS. The median follow-up time for surviving patients was 34.2 months (range: 2.3 - 38 months) in the Donor arm and 26.9 months (range: 2.4 - 37.2 months) in the No Donor arm.
Results
Enrollment
384 patients aged 50-75 with IPSS Int-2 or high-risk de novo MDS (Donor arm: n=260, No Donor arm: n=124) at 34 centers between 2014 and 2018
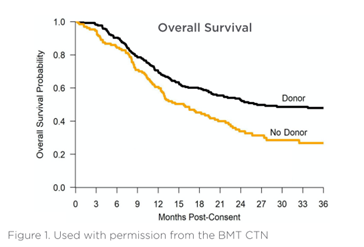
The Donor and No Donor arms were well balanced for age, gender, performance status, disease risk, MDS disease duration, and response to HMA therapy.
The adjusted Donor arm 3-year OS was 47.9% compared with 26.6% in the No Donor arm (p=0.0001) with an absolute difference of 21.3%.
Three-year LFS was greater in the Donor arm (35.8%) compared with the No Donor arm (20.6%, p=0.003).
These benefits were seen across all subgroups tested, including patients above or below Medicare age (>65). As treated analysis also demonstrated the benefit of HCT in those who underwent transplant as planned compared with patients who had no donor and did not proceed to transplant. No clinically significant differences in QOL were found.
Conclusions
The introduction of RIC regimens makes it possible for older patients with MDS to undergo alloHCT with improved outcomes compared to nontransplant therapy.
HCT referral should be offered to all individuals between the ages of 50-75 with high-risk MDS. Taking into account the Surveillance, Epidemiology, and End Results (SEER) data from 2013-2017, roughly 23,000 patients ages 50-74 are estimated to be candidates for transplant consultation each year.
The survival advantage observed within this older MDS population suggest that RIC HCT should rapidly become the standard of care.
Enhanced awareness and a wide adoption of early referral to HCT consultation may promote even higher HCT completion rates. The potential patient population with MDS that would benefit from this possible curative therapy versus supportive care is too large to ignore.
Improving access and changing practice
In 2019, the BMT CTN established a Task Force on Evidence into Practice to strengthen research dissemination and implementation for practice-changing studies like this one, working to identify and overcome barriers and health disparities.
The work of the Task Force is ongoing, as is the effort to use this and supporting data to expand Medicare coverage for HCT in MDS in collaboration with the CIBMTR® (Center for International Blood and Marrow Transplant Research®), National Marrow Donor Program®/Be The Match®, American Society for Transplantation and Cellular Therapy, and the American Society of Hematology.
The most important step in ensuring appropriate patients receive HCT at an optimal time is considering this curative option early.
Early referral to a transplant center enables timely identification of a suitable donor, assessment of candidacy for transplantation and other cellular therapy options, mitigation of potential co-morbidities, identification and resolution of insurance barriers, and coordination of care needs.
You can support this journey for your patient from pre- to post-transplant care by:
- Performing HLA typing at the time of diagnosis to start the donor search early and improve patient outcomes
- Referring early for a consult to discuss not just transplant, but other treatment options
- Coordinating with your transplant center to optimize post-treatment care at your practice
Access free HLA typing for your patients
HLA typing at diagnosis can increase the likelihood your patient can undergo HCT at the optimal time. Our HLA Today program removes barriers to patient and family member HLA typing. There is no cost to you or your patient – and no insurance paperwork to file.
Learn how you can make HLA Today part of your community oncology practice>
BMT CTN 1102 study: Related resources
References
Full study citation: Nakamura R, Saber W, Martens MJ, et al: Biologic assignment trial of reduced-intensity hematopoietic cell transplantation based on donor availability in patients 50-75 years of age with advanced myelodysplastic syndrome. J Clin Oncol 2021 Jun 9. Online ahead of print. DOI: 10.1200/JCO.20.03380.
Commentary on clinical practice: Warlick ED, Ustun C, Andreescu A, et al: Blood and Marrow Transplant Clinical Trials Network Study 1102 heralds a new era in hematopoietic cell transplantation in high-risk myelodysplastic syndromes: Challenges and opportunities in implementation. Cancer 2021 Aug 10. Online ahead of print. DOI: 10.1002/cncr.33826.
Recent support for study design and results: Gooley T: Two biologic-assignment studies evaluating the efficacy of hematopoietic cell transplant among older patients with high-risk myelodysplastic syndrome. J Clin Oncol 2021 Sep 7. Online ahead of print. DOI: 10.1200/JCO.21.01594.
