A report presented at the 2022 Tandem Meetings of the American Society of Transplantation and Cellular Therapy (ASTCT) and the CIBMTR® (Center for International Blood and Marrow Transplant Research®) from the National Marrow Donor Program® (NMDP)/Be The Match® shows that despite the challenges of the COVID-19 pandemic, hematopoietic cell transplantation (HCT) products can be reliably sourced and preserved without negative impacts to donors or patients. Cryopreserved products (Devine et al., 2021) and products from COVID-19 positive donors appear to pose no overt risk, and utilization challenges were adequately addressed after the start of the pandemic to ensure no major gaps in care for those in need of HCT.
The COVID-19 pandemic posed numerous logistical challenges in the way that the NMDP/Be The Match delivered allogeneic products to HCT patients. Prior to COVID-19, fresh bone marrow (BM) and peripheral blood stem cells (PBSCs) were used and infused into patients at the time of transplant in more than 90% of cases. Due to the pandemic, stem cell products could no longer be reliably delivered fresh and required cryopreservation. Thus, the NMDP/Be The Match pivoted to providing cryopreserved products. This research, therefore, sought to evaluate the effects of cryopreservation and the pandemic on the infusion of allogeneic products.
Data were assessed for all allogeneic products that were collected through the NMDP/Be The Match from March 17, 2020 – June 30, 2021. A total of 9,294 products were collected from both related and unrelated donors during the study period. Of these, a total of 8,700 products were infused both domestically and internationally.
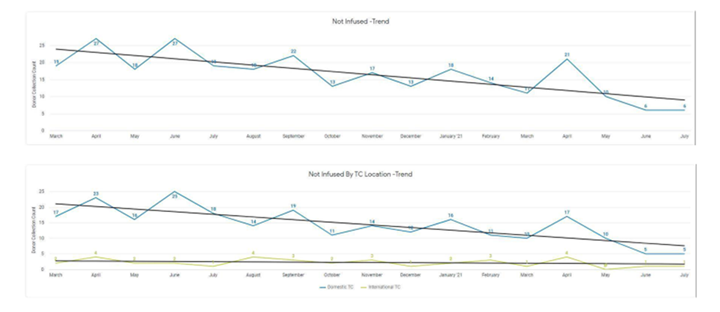
One of the main differences between fresh and cryopreserved products is that transplant centers can wait to infuse the product based on a variety of factors. At the time of this report, 374 products were pending infusion. However, over the last 18 months, there were only 220 products that were not infused. The number of products collected and infused on average per month was 544, while the number of products that were not infused each month averaged about 14. To evaluate changes in non-utilization over time, the first 5 months (March to July, 2020) were compared to the last 5 months (March to July, 2021) in the study period. As shown in the figure below, there is a significant downward trend, especially significant in domestic transplant centers.
There were 34 COVID-19 positive cases among donors either post or prior to donation. Of these cases, 17 recipients had a donor that tested positive post-donation (13 products were infused, and 4 were not infused based on the transplant center’s preference). Of the total cases, 13 had a donor with COVID-19 concerns that caused the collection to be stopped, and 4 were uncharacterized. Of the patients that had products infused, there were no known infections or deleterious effects on engraftment, even when the donor had been noted to have COVID-19 within a week of donation.
This report demonstrates that despite cryopreservation, there were a surprisingly low number of products that were not infused. Moreover, patients that received products from a COVID-19 positive donor did not become infected with COVID-19 nor suffered adverse effects. This data will help inform donors to aid them in making choices about donation as well as guide transplant centers and donor centers should there be future crises or instances when donor’s allogeneic products need to be cryopreserved. Products can be reliably sourced during a pandemic and process benefits from experiences gained resulting in lower non-utilization over time. Products from COVID-19 positive donor appear to be safe to use, with no overt risk to patients.
Change in Non-Utilization Over Time