Acute Myeloid Leukemia (AML) – Pediatric
Advances in understanding the cytogenetic and molecular abnormalities of acute myeloid leukemia (AML) have improved the ability to risk stratify both adult and pediatric patients to identify those who may benefit from hematopoietic cell transplantation (HCT).
Pediatric AML is a relatively rare disease, with an incidence of approximately 7 cases per million children younger than 15 years. [1]
Research demonstrates that allogeneic HCT in complete first remission for children and adults with high-risk AML results in outcomes comparable to those of standard-risk patients. [2] A 2017 multi-center study found that transplant survival in children with unfavorable karyotype AML has improved significantly over time due to decreased relapse risk. [3]
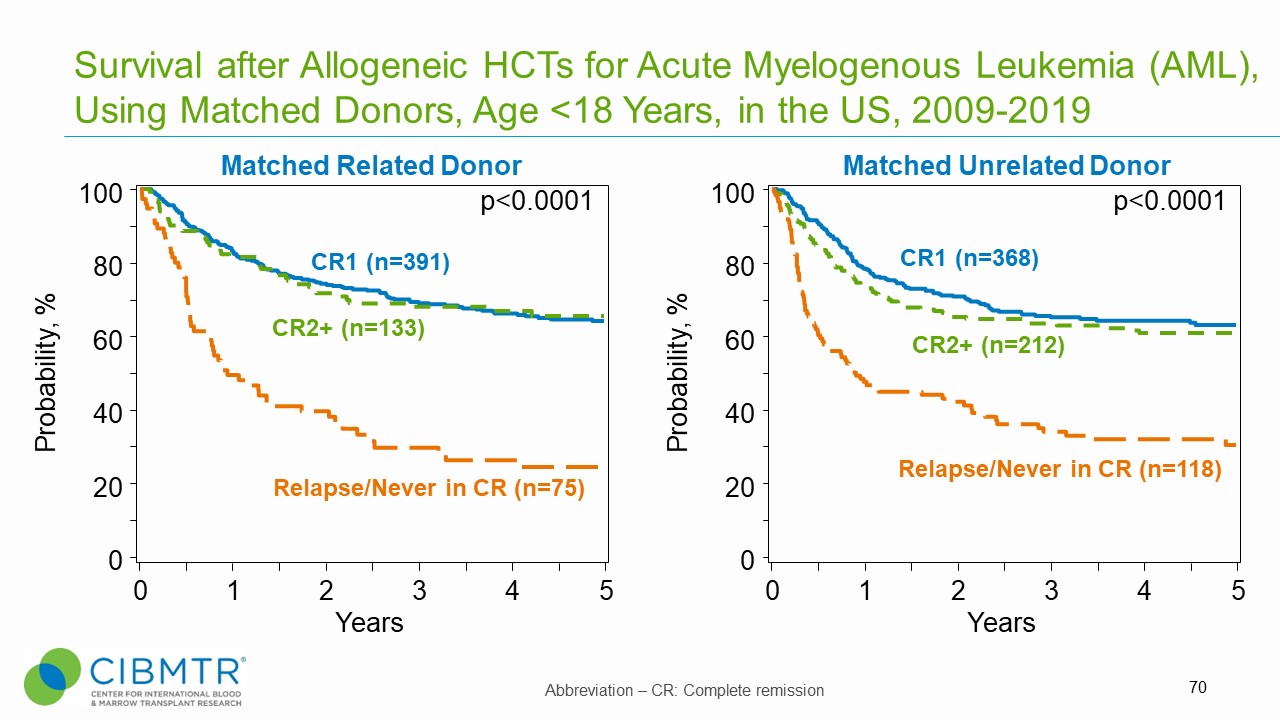
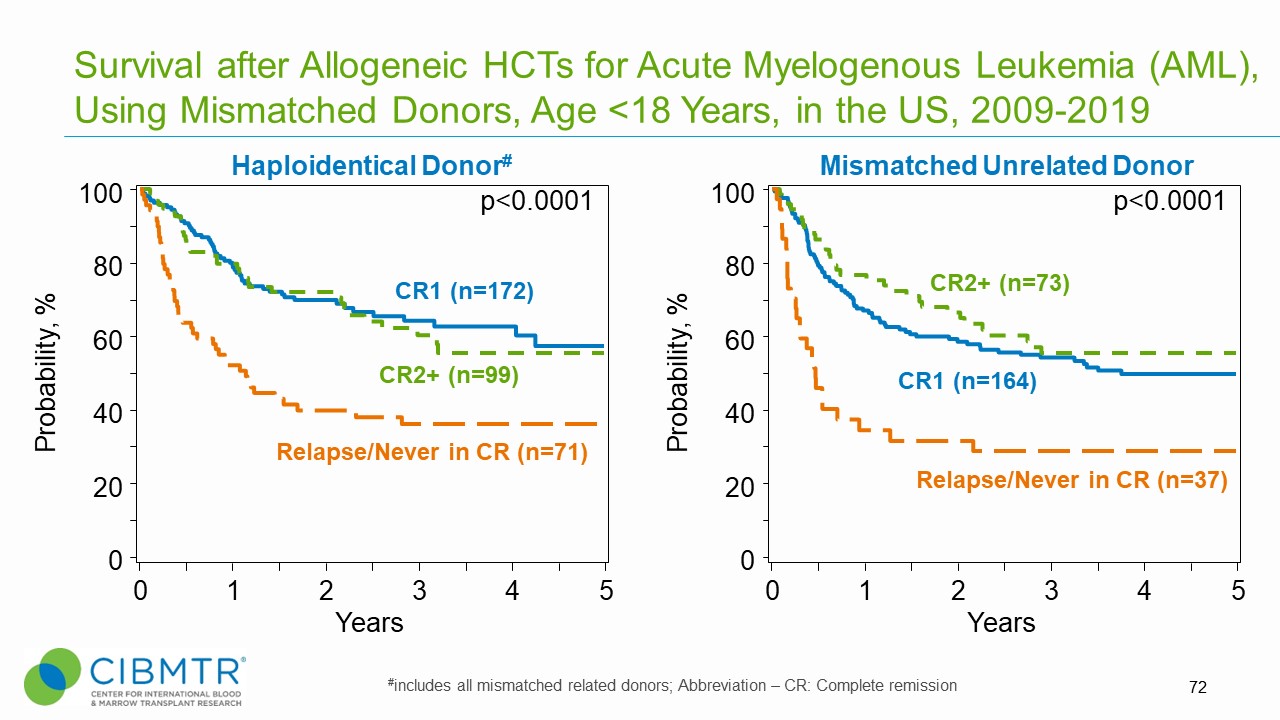
Pediatric AML patients who receive transplants in first or second complete remission (CR) experience significantly improved survival compared to those transplanted with advanced disease (primary induction failure, active disease, or CR3 and beyond).
Figure 1. Survival, Matched Related and Matched Unrelated HCT in Pediatric AML
Outcomes
Data in this section have been prepared by the CIBMTR® (Center for International Blood and Marrow Transplant Research®). The CIBMTR is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin.
Figure 2. Survival Over Time, Adult and Pediatric AML HCT
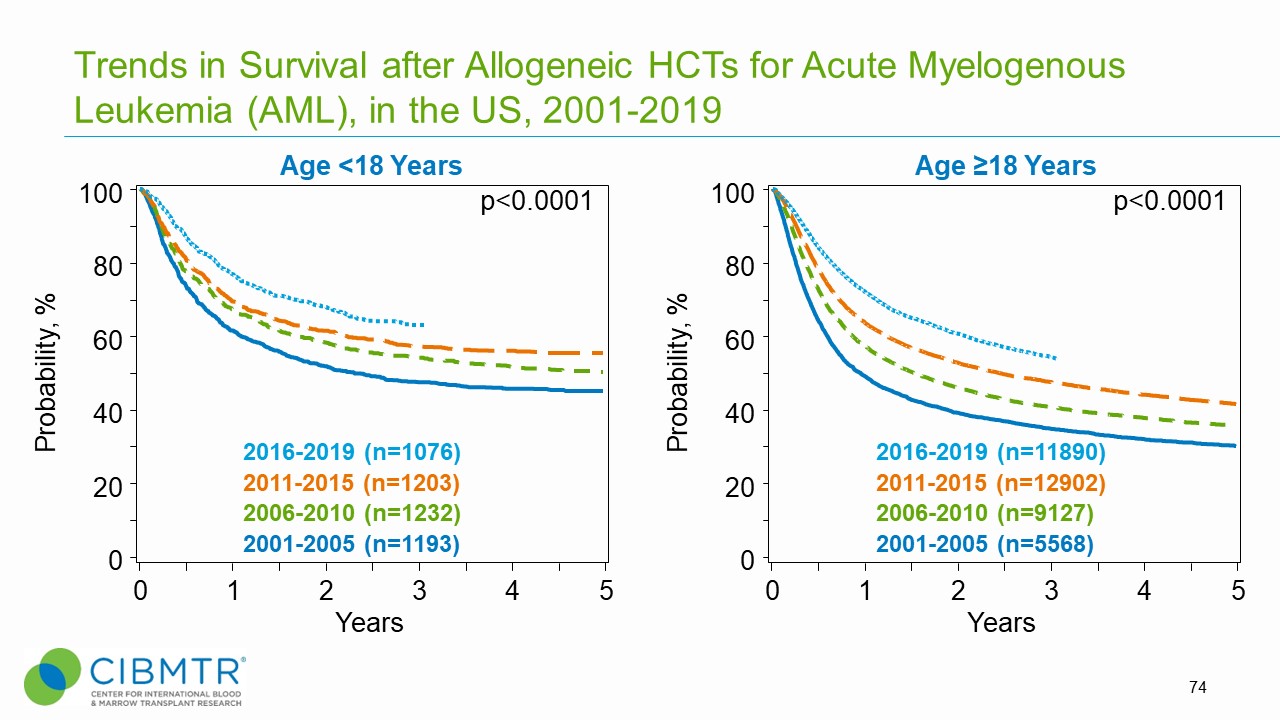
Figure 3. Survival Over Time, Haploidentical and Mismatched Unrelated HCT in Pediatric AML
HCT Consultation Timing Guidelines
he National Marrow Donor Program® (NMDP)/Be The Match® and the American Society for Transplantation and Cellular Therapy (ASTCT) have jointly developed guidelines for transplant consultation and referral timing based on disease characteristics. [4] The National Comprehensive Cancer Network Clinical Practice Guidelines (NCCN Guidelines®) were consulted when developing these guidelines and are a valuable tool in determining risk stratification. [5]
Our guidelines highlight disease categories that include patients at risk for disease progression and who should be referred for a consultation for autologous or allogeneic transplantation. [4]
Transplant Consultation Guidelines: Pediatric AML
High-resolution HLA typing is recommended at diagnosis for all patients
HCT consultation should take place early after initial diagnosis for all patients with AML, including:
- Age <2 years at diagnosis
- Primary induction failure
- Measurable (also called minimal) residual disease after initial therapy
- CR1 – except favorable risk AML [defined as: t(8;21)(q22;q21.1); RUNX1-RUNX1T1, inv(16)(p13. 1q22) or t(16;16)(p13. 1; q22); CBFB-MYH11, mutated NPM1 without FLT3-ITD or with FLT3-ITDlow, biallelic mutated CEBPA]
- Monosomy 5 or 7
- Treatment-related leukemia
- First relapse
- CR2 and beyond, if not previously evaluated
View complete HCT Consultation Timing Guidelines
Clinical Trials Search and Support
The NMDP/Be The Match offers the Be The Match® Jason Carter Clinical Trials Search and Support (CTSS) program, which can provide clinical trial navigation to your patients. The CTSS Program was created to help people with blood cancers or blood disorders and their families find and join clinical trials.
For more information, visit Clinical Trials Search and Support
References
- Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood. 2012; 120(16): 3187-3205. Access
- Burke MJ, Wagner JE, Cao Q, Ustun C, Verneris MR. Allogeneic hematopoietic cell transplantation in first remission abrogates poor outcomes associated with high-risk pediatric acute myeloid leukemia. Biol Blood Marrow Transplant. 2013; 19(7): 1021-1025. Access
- Alloin L-F, Leverger G, Dalle J-H, et al. Cytogenetics and outcome of allogeneic transplantation in first remission of acute myeloid leukemia: the French pediatric experience. Bone Marrow Transplant. 2017; 52(4): 516-521. Access
- NMDP/Be The Match and ASTCT Recommended Timing for Transplant Consultation. Download (PDF)
- National Comprehensive Cancer Network. Acute Myeloid Leukemia. (Version 2.2022). Access