Screening Eyes for Chronic GVHD
Below are clinical manifestations that are potential early indicators of chronic GVHD of the eyes. If GVHD is suspected, timely collaboration with the patient's transplant center is recommended to confirm the diagnosis and to develop and evaluate a treatment plan.
These guidelines are based on published diagnostic criteria from the National Institutes of Health (NIH) Consensus Development Project on chronic GVHD. [1,2,3]
Clinical Examination
- Visual inspection of the conjunctivae and sclerae
- Ophthalmologic exam
Diagnostic Testing
- Schirmer's tear test
- Slit-lamp examination
Patient-Reported Symptoms and Signs
- Dry, burning, gritty eyes
- Itching
- Orbital pain
- Difficulty opening eyes in the morning
- Sensitivity to light and wind
- Excessive tearing
- Diminished visual acuity and/or blurring
Possible Manifestations
New Onset Dry, Gritty, or Painful Eyes*
New ocular sicca documented by low Schirmer test values with a mean value of both eyes ≤ 5 mm of wetting at 5 minutes, but note that Schirmer’s test values are not useful for follow-up of ocular GVHD due to poor correlation with symptom change
Cicatricial Conjunctivitis*
Fibrous tissue scaring and inflammation
Keratoconjunctivitis Sicca (KCS)*
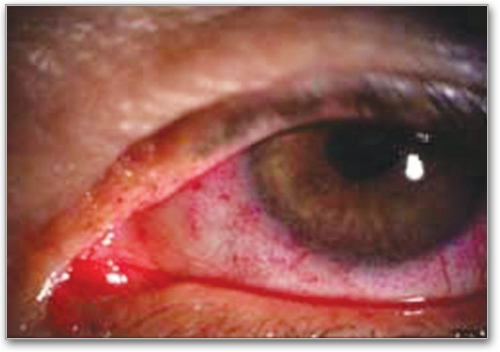
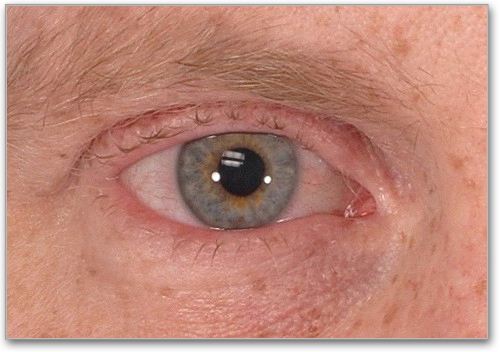
Inflammation of cornea and conjunctivae, with dryness, grittiness and/or orbital pain. Slit lamp exam with mean Schirmer's tear test values of 6 to 10 mm, not due to other causes
Confluent Areas of Punctate Keratopathy*
Closely spaced, non-inflamed pinpoint defects indicating loss of corneal epithelium, and observed with fluorescein staining
Photophobia**
Increased sensitivity to light
Periorbital Hyperpigmentation**
Excess pigmentation in the tissues surrounding or lining the orbit of the eye
Blepharitis**
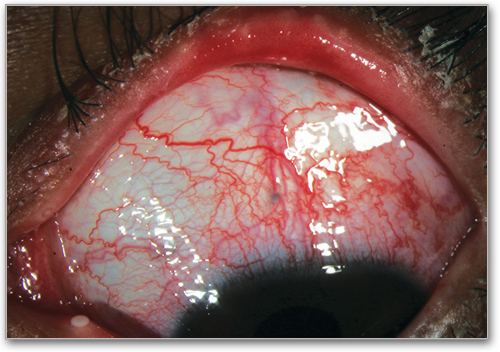
Erythema and edema of the eyelids and telangiectasia of lid margin
Notes
* Distinctive but insufficient alone to establish an unequivocal diagnosis of chronic GVHD without further testing or additional organ involvement.
** Rare, controversial, or non-specific features of chronic GVHD.
*** Common in both acute and chronic GVHD.
References
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2015; 21(3): 389-401.
- Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2015; 21(6): 984-999.
- These guidelines have been developed by the National Marrow Donor Program® (NMDP)/Be The Match® in consultation with Sandra A. Mitchell, CRNP, MScN, AOCN; National Institutes of Health Clinical Center; and Steven Z. Pavletic, M.D.; National Cancer Institute, National Institutes of Health, Bethesda, Md. The information in this document does not represent the official position of the NIH or the U.S. Government.
Additional review from:
- Dennis L. Confer, M.D., NMDP/Be The Match, Minneapolis, Minn.
- Linda J. Burns, M.D., NMDP/Be The Match, Minneapolis, Minn.
- Madan Jagasia, M.D., Vanderbilt University Medical Center, Nashville, Tenn.
- Stephanie J. Lee, M.D., Fred Hutchinson Cancer Research Center, Seattle, Wash.
Text adapted from reports of the NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease from Biology of Blood and Marrow Transplantation by American Society for Blood and Marrow Transplantation. Reproduced with permission of Elsevier, Inc.
Photo Credits
- Photos/ Keratosis Pilaris; Lichen Planus-like; Hypopigmentation; Sclerosis; Erosion; Maculopapular: Maria L. Turner, M.D.; Edward W.Cowen, M.D.; Dermatology Branch, National Cancer Institute, NIH, Bethesda, Md.
- Photos/ Poikiloderma; Morphea; Lichen Planus-like; Lichen Sclerosus-like; Hyperpigmentation; Sclerosis; Nail dystrophy; Alopecia; Edema: Edward W. Cowen, M.D.; Dermatology Branch, National Cancer Institute, NIH, Bethesda, Md.
- Photos/ Lichen planus; Mucoceles; Erythema: Mark M. Schubert, D.D.S., M.S.D.; Fred Hutchinson Cancer Research Center, Seattle, Wash.
- Photo/ Keratoconjunctivitis: Mary E.D. Flowers, M.D.; University of Washington, Seattle, Wash.
- Photo/ Blepharitis: Janine A. Smith, M.D.; National Eye Institute, NIH, Bethesda, Md.
All photos used with permission.