Acute Lymphoblastic Leukemia (ALL) - Pediatric
Approximately 6,000 individuals are diagnosed with acute lymphoblastic leukemia (ALL) each year in the United States. Children and adolescents younger than 20 years comprise about 54% of these cases, making ALL the most common type of pediatric cancer. [1]
ALL is the most common indication for allogeneic hematopoietic cell transplantation (HCT) in patients <18 years with hematological malignancies. [2] Research suggests that allogeneic HCT is recommended for pediatric ALL patients who experience primary induction failure, but subsequently achieve a first complete remission (CR1). Human leukocyte antigen (HLA) matched related and matched unrelated HCT provide equivalent outcomes.[3]
Outcomes
Data in this section have been prepared by the CIBMTR® (Center for International Blood and Marrow Transplant Research®). The CIBMTR is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin.
Figure 1. ALL Pediatric Survival, Matched Related and Matched Unrelated HCT
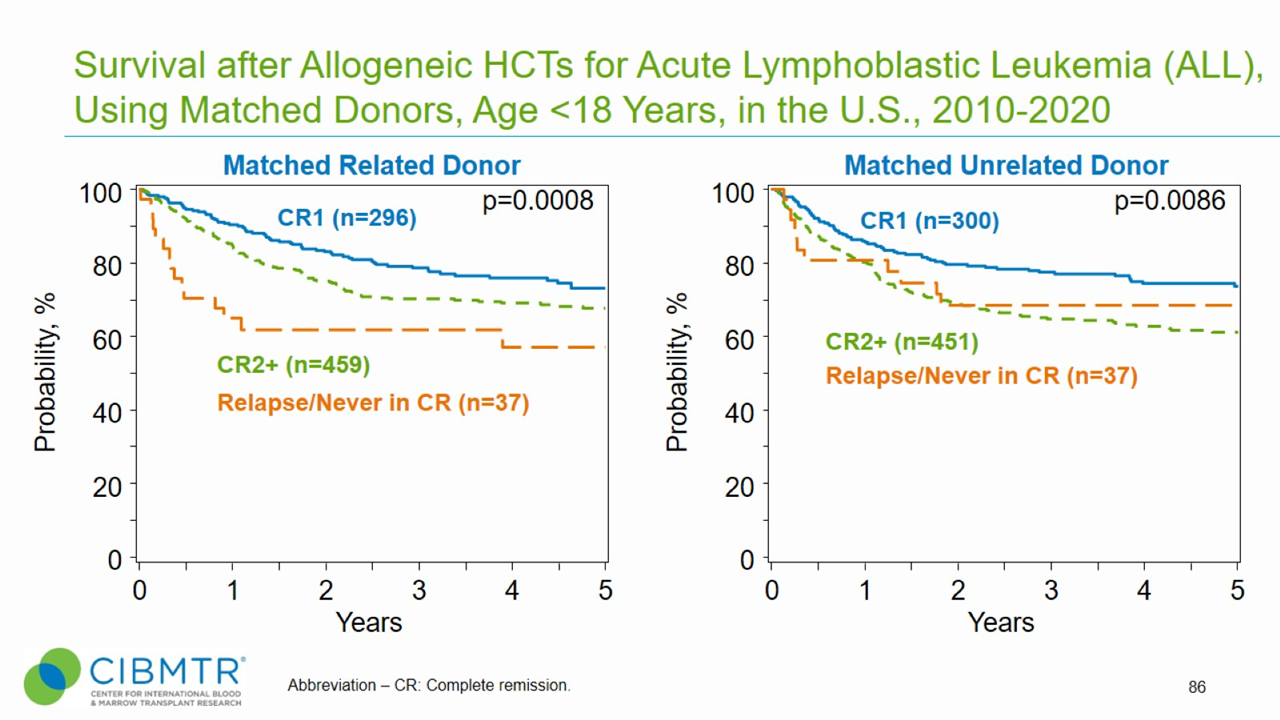
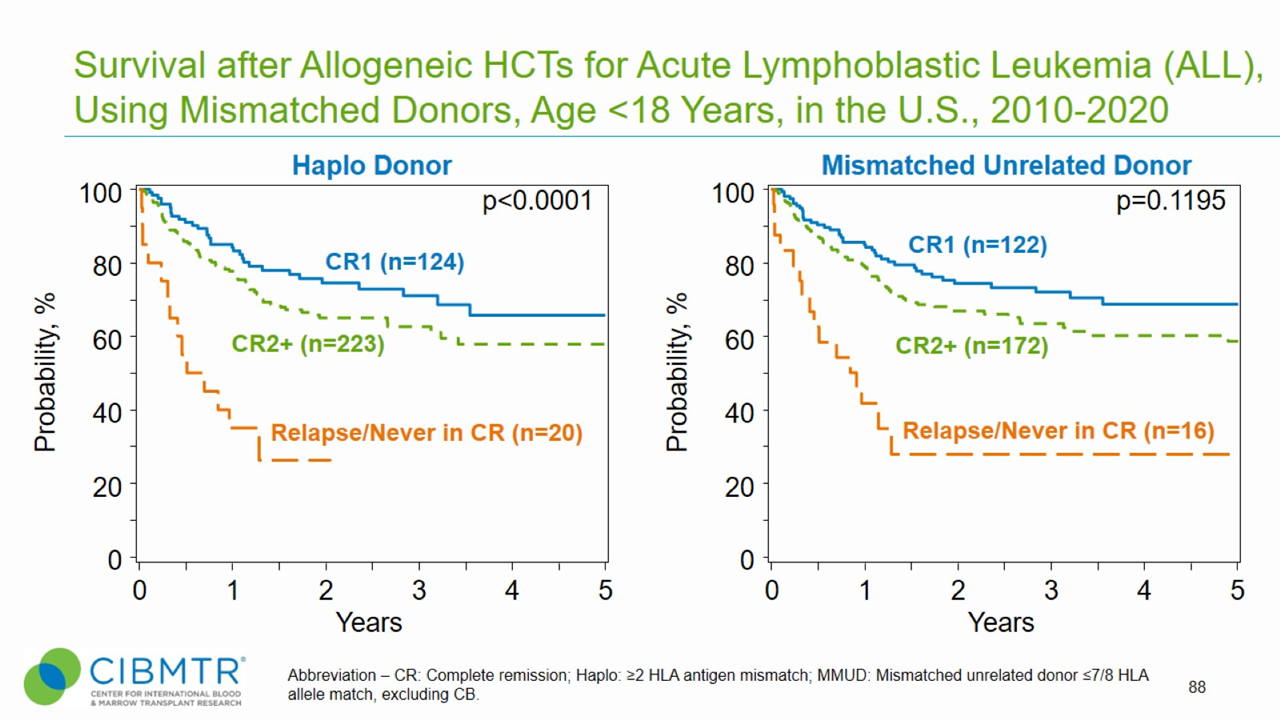
Figure 2. ALL Pediatric Survival, Haploidentical and Mismatched Unrelated HCT
HCT Consultation Timing Guidelines
The National Marrow Donor Program® (NMDP)/Be The Match® and the American Society for Transplantation and Cellular Therapy (ASTCT) have jointly developed guidelines for transplant consultation and referral timing based on disease characteristics. [4] The National Comprehensive Cancer Network Clinical Practice Guidelines (NCCN Guidelines®) were consulted when developing these guidelines and are a valuable tool in determining risk stratification. [5]
Our guidelines highlight patient and disease characteristics that put patients at risk for disease progression and who should be referred for a consultation for allogeneic transplantation. [4]
Transplant Consultation Guidelines: Acute Lymphoblastic Leukemia (ALL) - Age <15 years
High-resolution HLA typing is recommended at diagnosis for all patients
- Infant at diagnosis
- unfavorable genetics
- age <3 months with any WBC, or <6 months with WBC>300,000 at presentation
- Primary induction failure
- Presence of measurable (also known as minimal) residual disease after initial therapy
- High/very high-risk CR1, including:
- Philadelphia chromosome positive slow-TKI responders or with IKZF1 deletions; Philadelphia-like
- iAMP21
- 11q23 rearrangement
- First relapse
- CR2 and beyond, if not previously evaluated
- Chimeric Antigen Receptor Therapy (CAR-T)
Download slides
Transplant Consultation Guidelines: Acute Lymphoblastic Leukemia (ALL) - Age 15-39 Years
High-resolution HLA typing is recommended at diagnosis for all patients
- Primary induction failure
- Presence of measurable (also known as minimal) residual disease after initial therapy
- High/very high-risk CR1 including:
- Philadelphia chromosome positive or Philadelphia-like
- iAMP21
- 11q23 rearrangement
- B-cell with poor-risk cytogenetics
- First relapse
- CR2 and beyond, if not previously evaluated
Download slides
View complete HCT Consultation Timing Guidelines
CAR-T Cell Therapy Video for Patients
Chimeric antigen receptor T cell (CAR-T) therapy for treating relapsed/refractory pediatric and young adult patients with B-cell ALL received FDA approval in 2017. [6]
In this easy-to-understand video, Linda J. Burns, M.D., former Vice President, Health Services Research, and Scott Kerwin, R.N., M.N., C.C.R.C., C.C.R.N., former Clinical Trial Patient Education Specialist at the NMDP/Be The Match, explain what CAR-T cell therapy is, who it may help, what the treatment is like, potential risks and benefits, questions to ask your doctor, and more. View the video and share it with patients and caregivers who are looking for information on CAR-T therapy as a treatment option.
Clinical Trials Search and Support
The NMDP/Be The Match offers the Be The Match® Jason Carter Clinical Trials Search and Support (CTSS) program, which can provide clinical trial navigation to your patients. The CTSS Program was created to help people with blood cancers or blood disorders and their families find and join clinical trials.
For more information, visit Clinical Trials Search and Support.
References
- SEER Stat Fact Sheets: Acute Lymphocytic Leukemia. Accessed 1 November, 2017. Access
- D'Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2017. Available at: http://www.cibmtr.org
- Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: Update of the 2005 Evidence-Based Review. Biol Blood Marrow Transplant. 2012; 18(4): 505-522. Access
- NMDP/Be The Match and ASTCT Recommended Timing for Transplant Consultation. Download (PDF)
- National Comprehensive Cancer Network. Pediatric Acute Lymphoblastic Anemia. (Version 1.2023). Access
- Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B-cell ALL (ELIANA) (ClinicalTrials.gov identifier: NCT02435849). Access