Non-Hodgkin Lymphoma (NHL)
Non-Hodgkin lymphomas (NHL) are a highly heterogeneous group of lymphoproliferative disorders originating in B lymphocytes, T lymphocytes or natural killer (NK) cells. In the United States, B-cell NHL represents 80-85% of cases; T-cell NHL, 15-20%; and NK NHL is rare. Most hematopoietic cell transplantation (HCT) performed for NHL is autologous, but in some cases, physicians may perform allogeneic HCT for particularly high-risk relapsed or refractory disease. [1, 2]
Figure 1 shows the increase by age group in the number of unrelated donor transplants facilitated by the National Marrow Donor Program® (NMDP)/Be The Match®, including patients with NHL.
Figure 1. Trend of Allogeneic HCT for Malignant Diseases in Adult and Pediatric Recipients

Outcomes
Data in this section have been prepared by the CIBMTR® (Center for International Blood and Marrow Transplant Research®). The CIBMTR is a research collaboration between the NMDP/Be The Match and the Medical College of Wisconsin.
Figure 2. Allogeneic HCT Volume Over Time
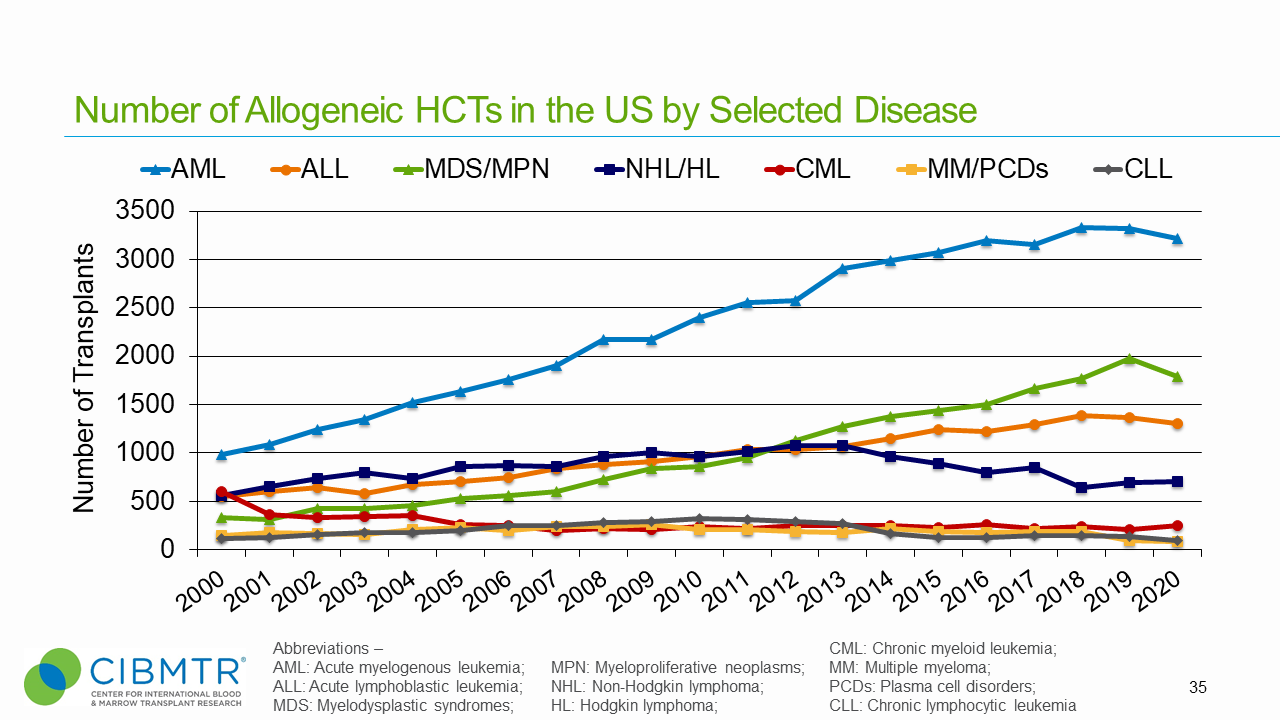
Figure 3. U.S. Volume of Autologous HCT Over Time
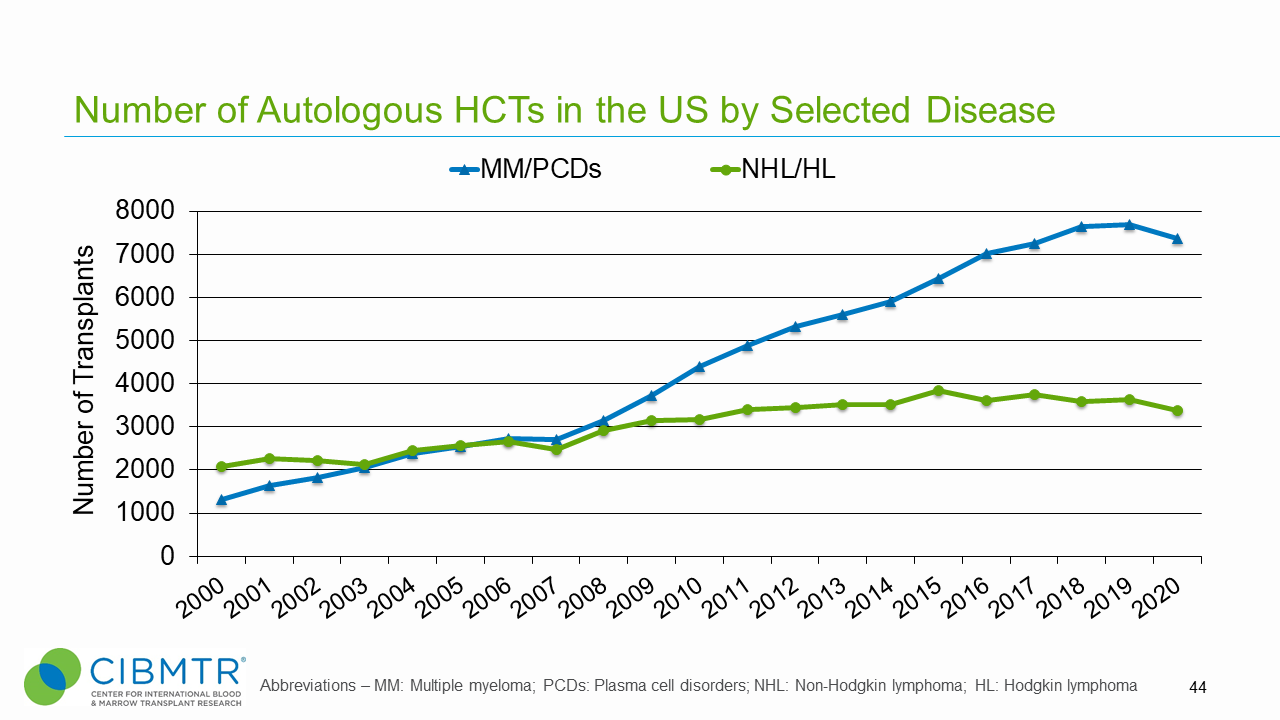
Figure 4. U.S. Volume of Autologous Diffuse Large B-Cell Lymphoma (DLBCL) HCT
 HCT.png)
Figure 5. Survival, Autologous or Allogeneic Follicular Lymphoma (FL) HCT
 HCT.png)
Figure 6. Survival Trends, Autologous FL HCT Over Time
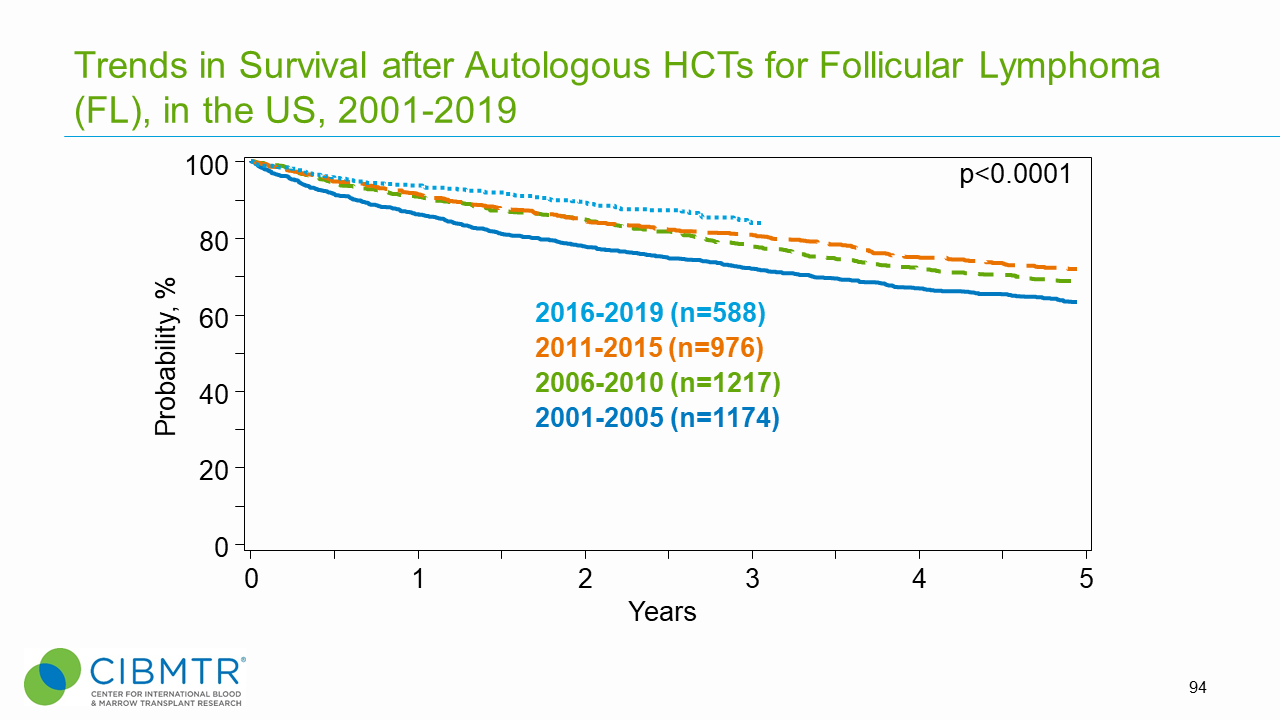
Figure 7. Survival Trends, Autologous DLBCL HCT Over Time
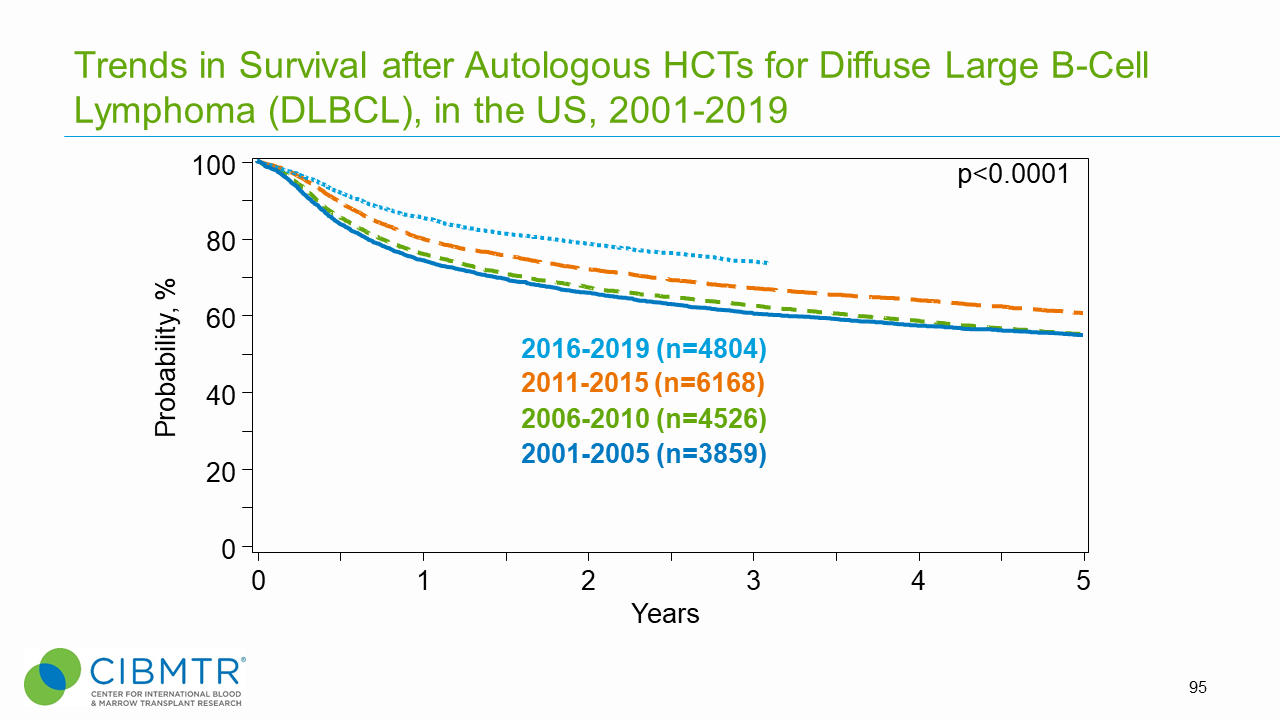
Figure 8. Survival, Autologous or Allogeneic Mantle Cell Lymphoma (MCL) HCT
 HCT.png)
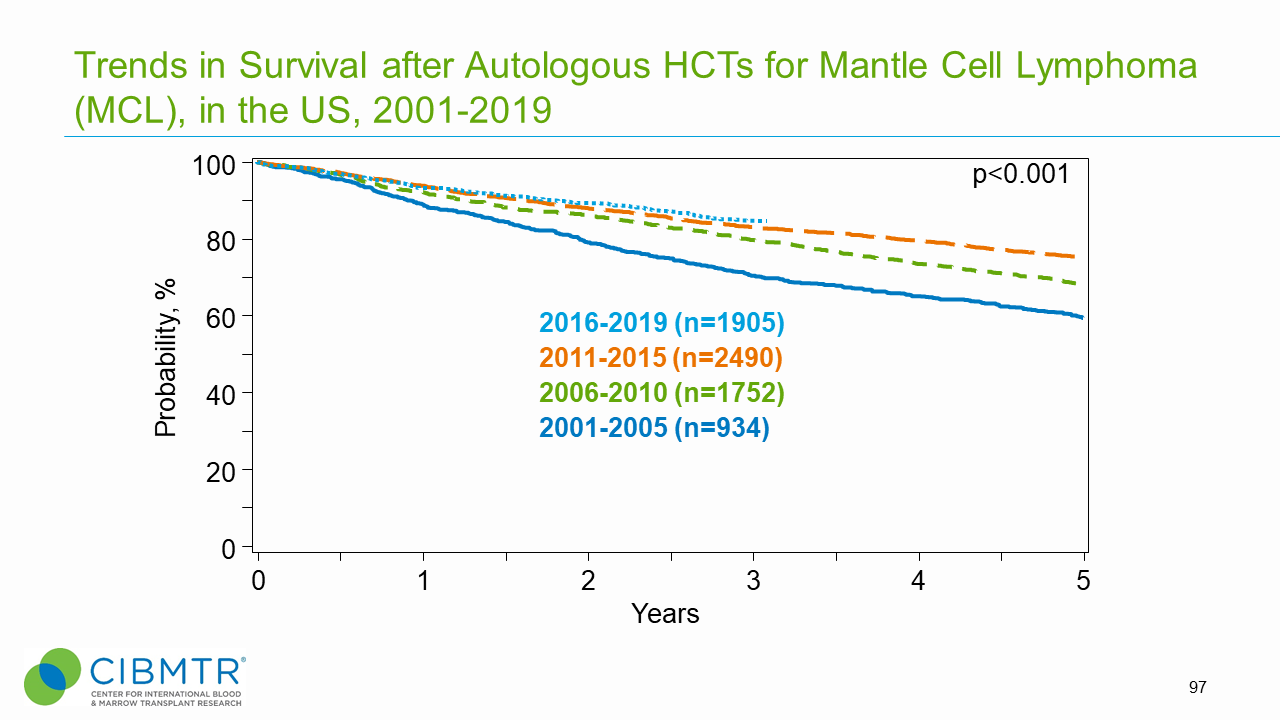
Figure 9. Survival Trends, Autologous MCL HCT
HCT Consultation Timing Guidelines
The National Marrow Donor Program® (NMDP)/Be The Match® and the American Society for Transplantation and Cellular Therapy (ASTCT) have jointly developed guidelines for transplant consultation and referral timing based on disease characteristics. [1] The National Comprehensive Cancer Network Clinical Practice Guidelines (NCCN Guidelines®) were consulted in developing these guidelines and are a valuable tool in determining risk stratification. [3]
Our guidelines highlight disease categories that include patients at risk for disease progression and who should be referred for a consultation for autologous or allogeneic transplantation. [1]
Transplant Consultation Guidelines: NHL
Follicular Lymphoma
- Poor response to initial treatment
- Initial remission duration <24 months
- First relapse
- Transformation to diffuse large B-cell lymphoma
Diffuse Large B-Cell Lymphoma
- Primary induction failure, including residual PET avid disease
- First relapse
- CR2 or subsequent remission
- Double or triple hit (MYC and BCL-2 and/or BCL-6) – at diagnosis
- Primary CNS lymphoma at diagnosis PIF or first relapse
High Grade B-Cell Lymphoma
- MYC and BCL-2 and/or BCL-6 rearrangements
- Primary induction failure
- CR1
- First relapse
- CR2 or subsequent remission
Mantle T-Cell Lymphoma
- At diagnosis
- First relapse
- Bruton's tyrosine kinase (BTK) intolerant or resistant disease
Mature T-Cell Lymphoma
- CR1
- First relapse
Other High-Risk Lymphomas
- At diagnosis
View complete HCT Consultation Timing Guidelines
CAR-T Cell Therapy Video for Patients
Chimeric antigen receptor T-cell (CAR-T) therapy is a new treatment focus for hematologic malignancies. Though some limitations are a barrier, new research shows promising safety and efficacy with targeted gene therapy for the treatment of NHL. [4]
In this easy-to-understand video, Linda J. Burns, MD, former Vice President, Health Services Research, and Scott Kerwin, RN, MN, CCRC, CCRN, former Clinical Trial Patient Education Specialist at the NMDP/Be The Match, explain what CAR-T therapy is, who it may help, what the treatment is like, potential risks and benefits, questions to ask your doctor, and more. View the video and share it with patients and caregivers who are looking for information on CAR-T therapy as a treatment option.
Clinical Trials Search and Support
The NMDP/Be The Match offers the Be The Match® Jason Carter Clinical Trials Search and Support (CTSS) program, which can provide clinical trial navigation to your patients. The CTSS Program was created to help people with blood cancers or blood disorders and their families find and join clinical trials.
For more information, visit Clinical Trials Search and Support.
References
- National Comprehensive Cancer Network. B Cell Lymphomas. (Version 5.2022). Access
- Dreyling M, Ferrero S, Hermine O. How to manage mantle cell lymphoma. Leukemia. 2014; 28(11): 2117-2130. Access
- NMDP/Be The Match and ASTCT Recommended Timing for Transplant Consultation. Download (PDF)
- Zhang J., Hu Y., Yang J. et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022; 609: 369–374. Access