Hodgkin Lymphoma
Approximately 8,500 individuals are diagnosed with Hodgkin lymphoma (HL) each year in the United States. HL is most frequently diagnosed among people aged 20-34 years. [1] Hematopoietic cell transplantation (HCT), often autologous but occasionally allogeneic, is a treatment option for recurrent disease. Allogeneic HCT outcomes in patients with relapsed/refractory HL have significantly improved over time. [2,3]
Outcomes
Data in this section have been prepared by the CIBMTR® (Center for International Blood and Marrow Transplant Research). The CIBMTR is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin.
Figure 1. HL Survival, Autologous and Allogeneic HCT
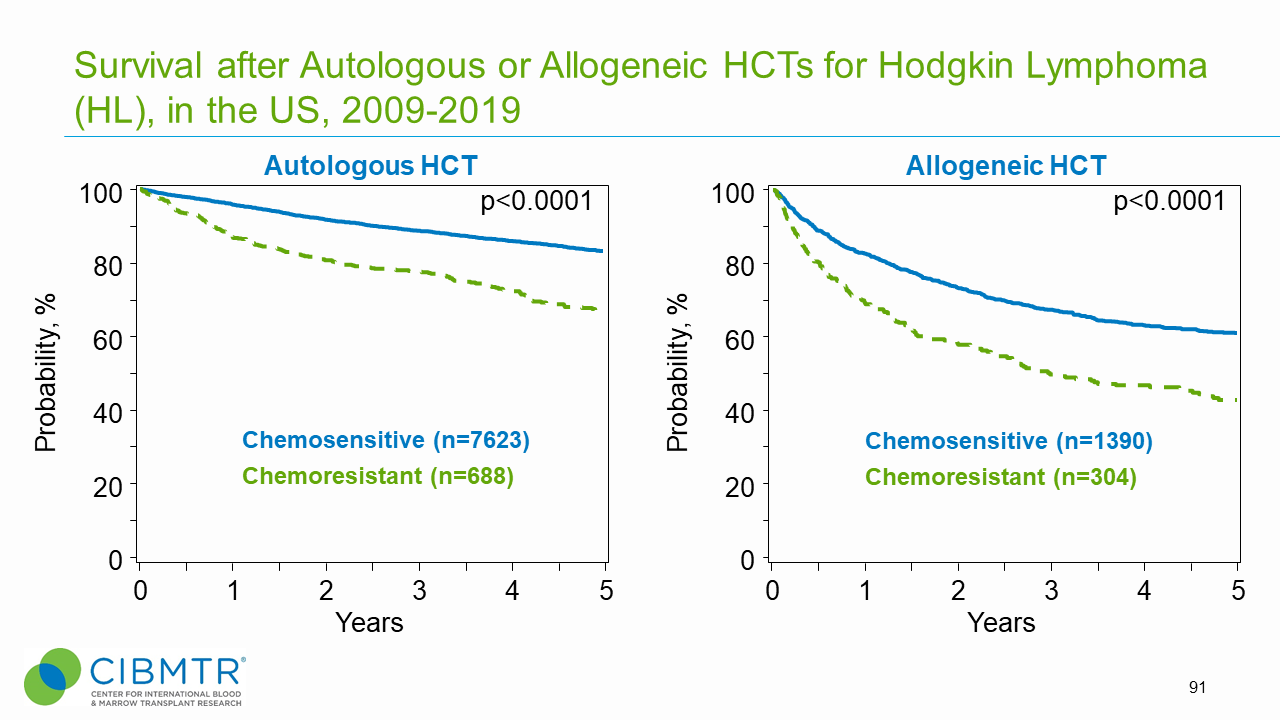
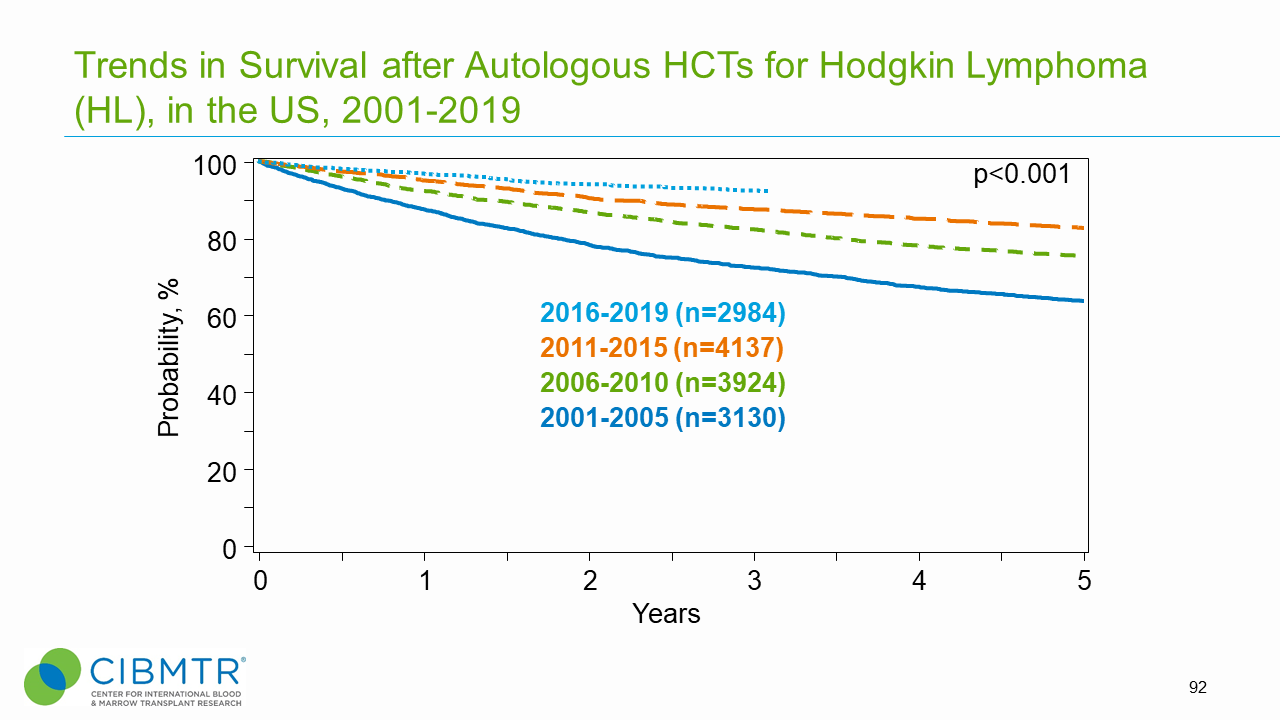
Figure 2. Survival Trends Over Time, HL HCT
HCT Consultation Timing Guidelines
The National Marrow Donor Program® (NMDP)/Be The Match® and the American Society for Transplantation and Cellular Therapy (ASTCT) have jointly developed guidelines for transplant consultation and referral timing based on disease characteristics. [4] The National Comprehensive Cancer Network Clinical Practice Guidelines (NCCN Guidelines®) were consulted when developing these guidelines and are a valuable tool in determining risk stratification. [2]
Our guidelines highlight disease categories that include patients at risk for disease progression and who should be referred for a consultation for autologous or allogeneic transplantation. [4]
Transplant Consultation Guidelines: Hodgkin Lymphoma
- Primary induction failure
- First relapse
- CR2 or subsequent relapse
Download as a slide
View complete HCT Consultation Timing Guidelines
Clinical Trials Search and Support
The NMDP/Be The Match offers the Be The Match® Jason Carter Clinical Trials Search and Support (CTSS) program, which can provide clinical trial navigation to your patients. The CTSS Program was created to help people with blood cancers or blood disorders and their families find and join clinical trials.
For more information, visit Clinical Trials Search and Support.
References
- SEER Stat Fact Sheets: Hodgkin Lymphoma. Website accessed 2 November, 2017. Access
- National Comprehensive Cancer Network. Hodgkin Lymphoma. (Version 2.2023). Access
- Hegerova L, Cao Q, Lazaryan A, et al. Improving outcomes after allogeneic hematopoietic cell transplantation for Hodgkin lymphoma in the brentuximab vedotin era. Bone Marrow Transplant. 2017; 52(5): 697-703. Access
- NMDP/Be The Match and ASTCT Recommended Timing for Transplant Consultation. Download (PDF)