Myelodysplastic Syndromes (MDS) - Hematopoietic Cell Transplant Survival Data & Advances
Approximately 21,000 people are diagnosed with myelodysplastic syndromes (MDS) in the United States each year, and the incidence increases with age. [1] More MDS patients are now eligible for allogeneic hematopoietic cell transplantation (HCT) than ever before. The development of reduced-intensity conditioning regimens makes HCT—which is the only potentially curative treatment option—more feasible for older patients. [2]
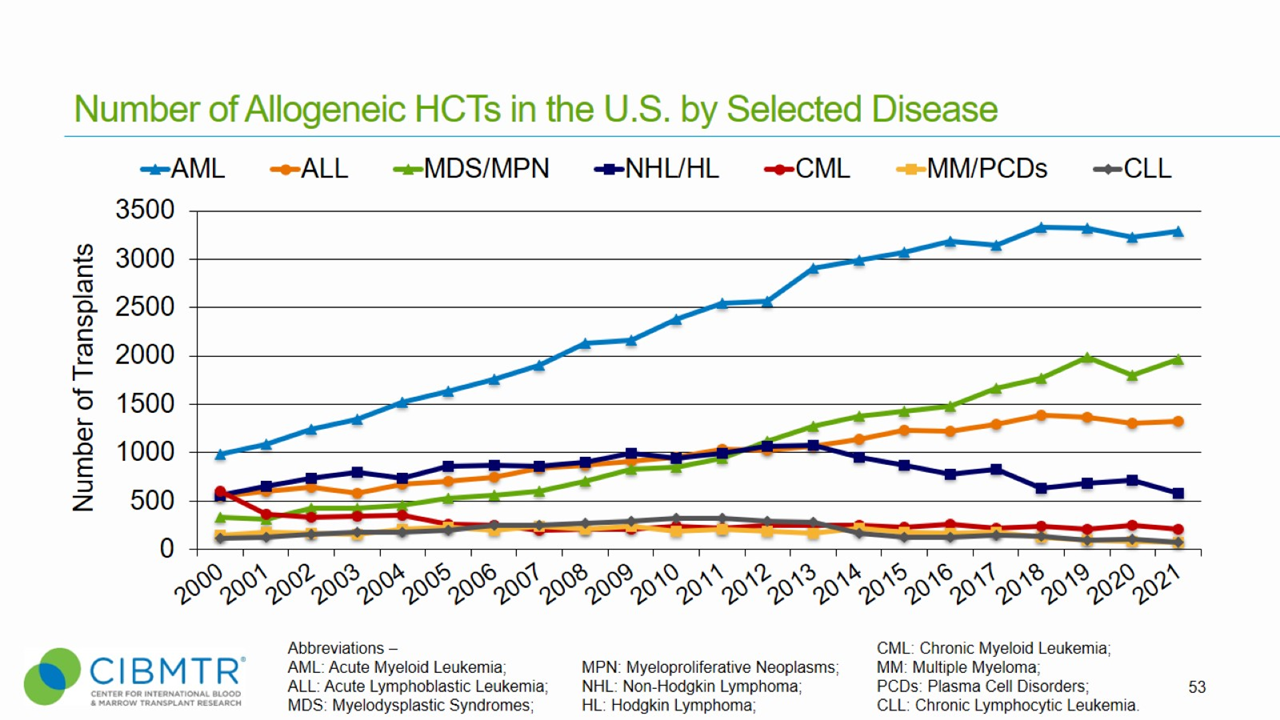
Figure 1 shows that annual transplants for MDS have steadily increased, making MDS the second most common indication for unrelated donor transplants facilitated by the National Marrow Donor Program® (NMDP)/Be The Match®.
Figure 1. HCT Volume Over Time in the U.S.
The Centers for Medicare and Medicaid Services (CMS) cover transplants for MDS when performed as part of a Medicare approved clinical study, which allows physicians to offer transplant as an option for older patients. Physicians can enroll Medicare-eligible patients with MDS in a CMS-approved study conducted by the CIBMTR® (Center for International Blood and Marrow Transplant Research®).
Preliminary results from the CIBMTR clinical trial showed that in patients who are eligible for allogeneic HCT, there was no difference in 100-day mortality or overall survival at 2 years for patients age 55-64 years compared to patients age 65 years and older. [3]
Research from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) published in the Journal of Clinical Oncology demonstrates that HCT provides a significant survival benefit for older patients with MDS. BMT CTN 1102 was a multi-center biologic assignment trial comparing reduced-intensity allogeneic HCT to hypomethylating therapy or best supportive care in patients aged 50-75 with advanced MDS. [4]
Outcomes
Data in this section have been prepared by the CIBMTR. The CIBMTR is a research collaboration between the NMDP/Be The Match and the Medical College of Wisconsin.
Figure 2. Survival, Adult MDS Matched Related and Matched Unrelated HCT
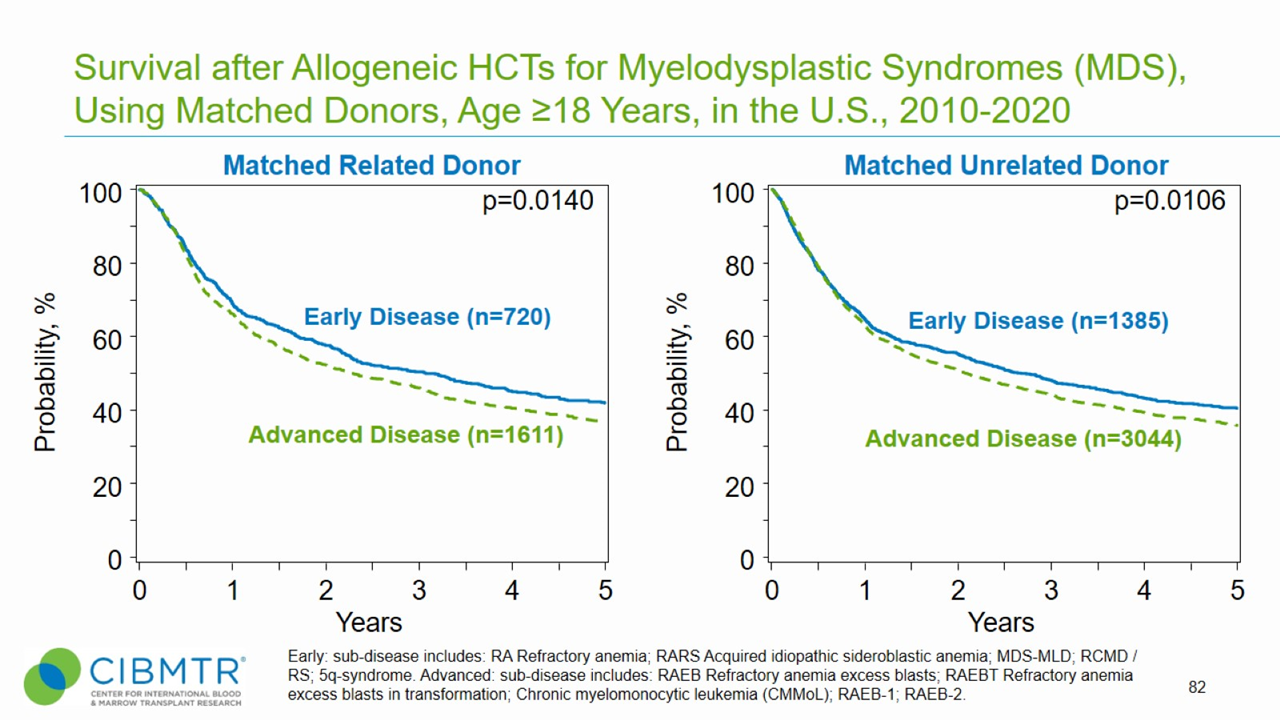
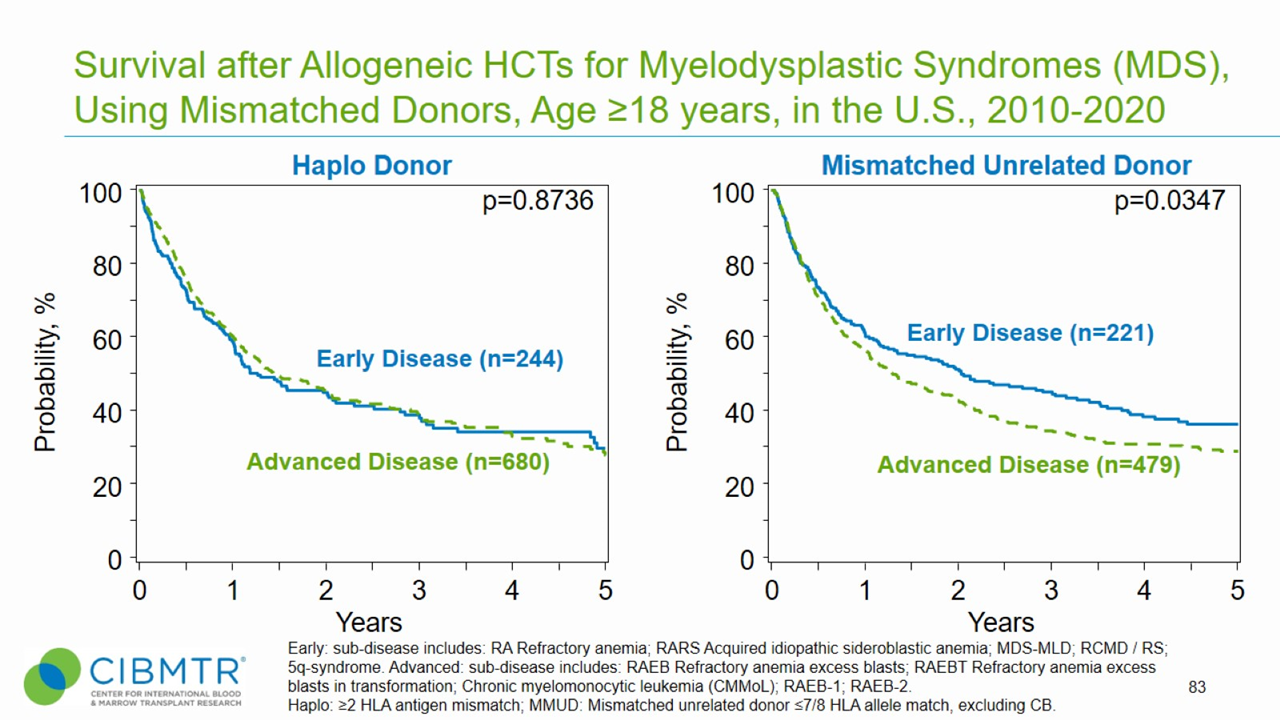
Figure 3. Survival, Adult MDS Haploidentical and Mismatched Unrelated HCT
Figure 4. Age Trend of Allogeneic HCT
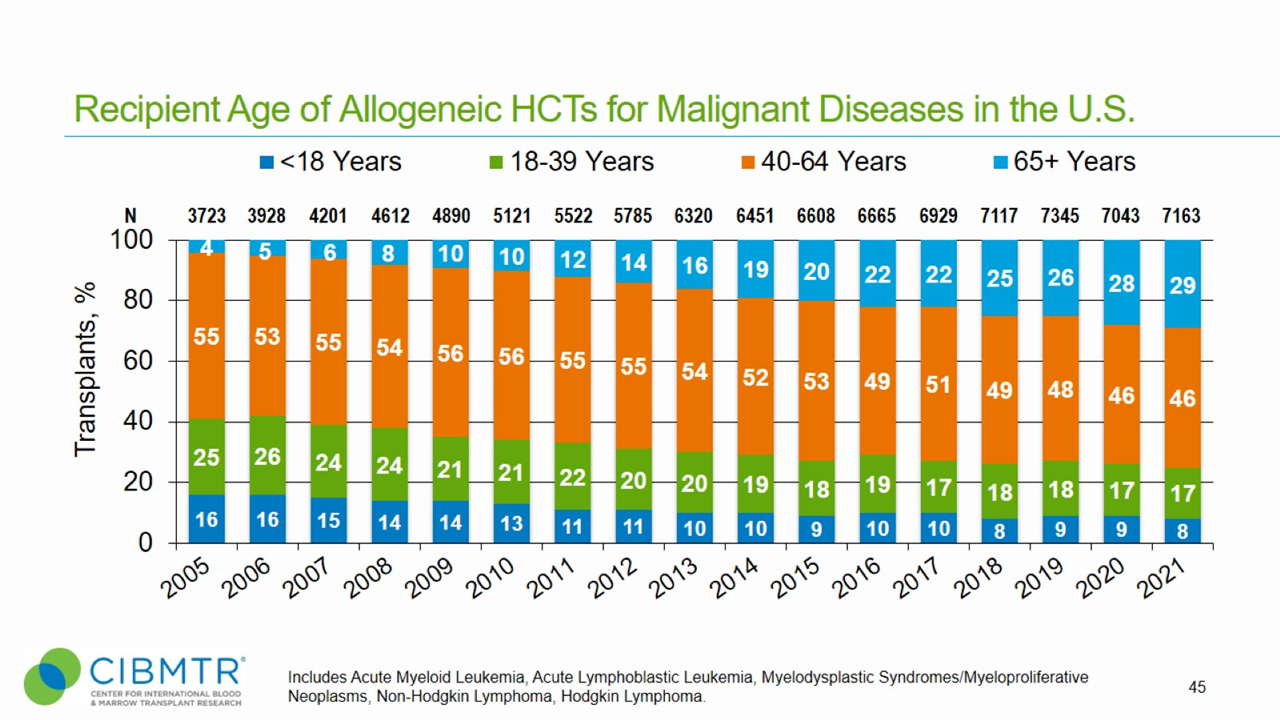
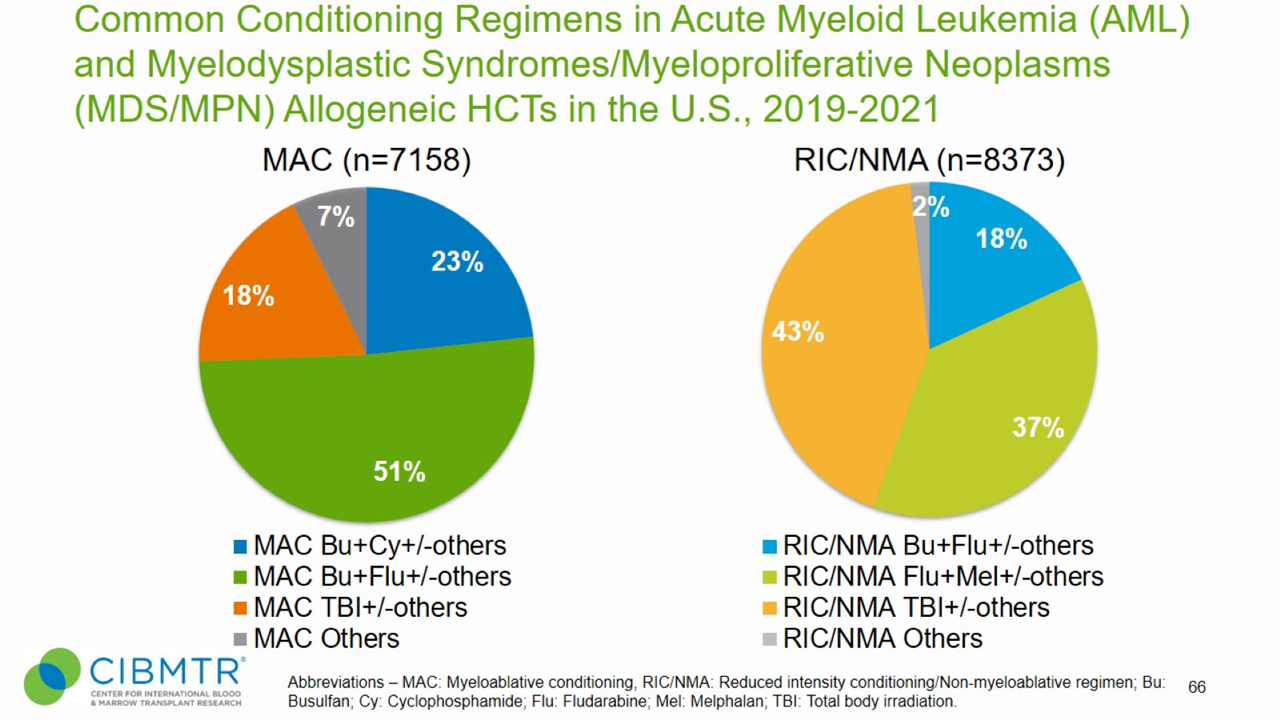
Figure 5. Conditioning Regimens for Allogeneic HCT, MDS
HCT Consultation Timing Guidelines
The National Marrow Donor Program® (NMDP)/Be The Match® and the American Society for Transplantation and Cellular Therapy (ASTCT) have jointly developed guidelines for transplant consultation and referral timing based on disease characteristics. [5] The National Comprehensive Cancer Network Clinical Practice Guidelines (NCCN Guidelines®) were consulted when developing these guidelines and are a valuable tool in determining risk stratification. [6]
Our guidelines highlight disease categories that include patients at risk for disease progression and who should be referred for a consultation for autologous or allogeneic transplantation [5].
Transplant Consultation Guidelines: Myelodysplastic Syndromes – Adult
High-resolution HLA typing is recommended at diagnosis for all patients
Any intermediate or high IPSS or IPSS-R score
Any MDS with poor prognostic features including:
- Treatment-related MDS
- Refractory cytopenias
- Adverse cytogenetics and molecular features
- Transfusion dependence
- Failure of hypomethylating agents or chemotherapy
- Moderate to severe marrow fibrosis
Download as a slide
Transplant Consultation Guidelines: Myelodysplastic Syndromes – Pediatric
Pediatric Myelodysplastic Syndromes
- At diagnosis for all subtypes
Download as a slide
Juvenile Myelomonocytic Leukemia (JMML)
- At diagnosis
Download as a slide
View complete HCT Consultation Timing Guidelines
Clinical Trials Search and Support
The NMDP/Be The Match offers the Be The Match® Jason Carter Clinical Trials Search and Support (CTSS) program, which can provide clinical trial navigation to your patients. The CTSS Program was created to help people with blood cancers or blood disorders and their families find and join clinical trials.
For more information, visit Clinical Trials Search and Support.
References
- SEER Cancer Statistics Review 1975-2012: MDS. Website accessed 8 March, 2018. Access
- Karanes C, Nelson GO, Chitphakdithai P, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008; 14(9, Suppl.): 8-15. Access
- Atallah E, Horowitz MM, Logan B, et al. Outcome of patients 65 years and older with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation compared to patients 55-64 years of age (abstract). Blood. 2015; 126(23): 193. Access
- Nakamura R, Saber W, Martens MJ, et al. Biologic assignment trial of reduced-intensity hematopoietic cell transplantation based on donor availability in patients 50-75 years of age with advanced myelodysplastic syndrome. Journal of Clinical Oncology. 2021;39(30):3328-3339. Access
- NMDP/Be The Match and ASTCT Recommended Timing for Transplant Consultation. Download (PDF)
- National Comprehensive Cancer Network. Myelodysplastic Syndromes. (Version 1.2023). Access