According to research presented at the European Group for Blood and Marrow Transplantation (EBMT) Annual Meeting, a National Marrow Donor Program® (NMDP)/Be The Match® sponsored phase II multi-center clinical trial shows outcomes remain very good at 3 years for patients receiving a mismatched unrelated donor hematopoietic stem cell transplant (MMUD HCT) with post-transplant cyclophosphamide (PTCy). Patients receiving reduced-intensity conditioning (RIC) in particular have excellent survival and very low rates of chronic graft-versus-host disease (cGVHD). These outcomes highlight the potential for MMUD HCT to expand access to HCT, especially for those who are racially and ethnically diverse that may not easily find a fully matched donor.
Download a PDF of the full study summary with journal citation here:
The use of PTCy for GVHD prevention has resulted in reductions in GVHD and improved outcomes in allogeneic HCT, especially in cases with mismatched donors. To evaluate the use of PTCy in MMUD HCT as a tool to expand HCT access, the CIBMTR® (Center for International Blood and Marrow Transplant Research®) performed a multi-center phase II prospective clinical trial using PTCy in MMUD called 15-MMUD. The study met its primary endpoint of greater than 65% overall survival (OS) at 1 year with an excellent OS of 76% [Shaw et al., 2021]. The 3-year outcomes in this study cohort were reported at EBMT in March 2022.
The median patient age was 52 (18-70) years old and a remarkable 48% of patients were from racial or ethnic minority groups. HLA matching was between 4 – 6/8, considering high resolution matching at HLA-A, B, C and DRB1, in 39% of transplants (43% in the RIC cohort, 39% in the myeloablative conditioning [MAC] cohort) and 61% of transplants were in 7/8 matches. 3-year outcomes are shown in Table 1 below.
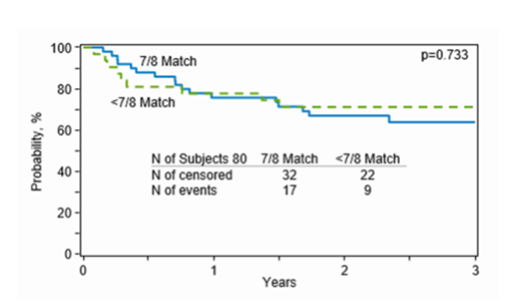
OS in the RIC cohort was particularly good at 70%. Non-relapse mortality was 15%. Rates of cGVHD were low at 20% for all grades and 5% for severe cGVHD, with a relapse rate of 29%. GVHD-/relapse-free survival (GRFS) was 44%. Although 3-year survival in the MAC cohort remained acceptable at 62%, relapse rates were high at 51%. Non-relapse mortality was 10%. cGVHD occurred in 38% of patients, with 13% reporting severe disease. GRFS in this cohort was correspondingly low at 17%. OS was 63% in the 7/8 cohort and 71% in the less than 7/8 (4-6/8) cohort (see Figure 1 below).
Outcomes for patients receiving a MMUD HCT using PTCy-based GVHD prevention remain very good with follow up at 3 years post-transplant. Patients receiving RIC in particular have excellent survival and very low rates of cGVHD. Use of more mismatched donors ( <7/8) was not associated with worse outcomes, providing early reassurance that access to transplant can be safely expanded to patients with no 7 or 8/8 matched donors, which is more common in patients who are racially and ethnically diverse. The NMDP/Be The Match and the CIBMTR are committed to expanding access to all patients in need of HCT (this study acts as a precursor to the ACCESS trial, which is currently enrolling patients). Our research programs are developing and evaluating novel treatment strategies that allow for the safe and effective use of MMUD HCT, expanding access to 100% of patients in need with excellent outcomes.
Table 1: 1 and 3-Year Survival Outcomes
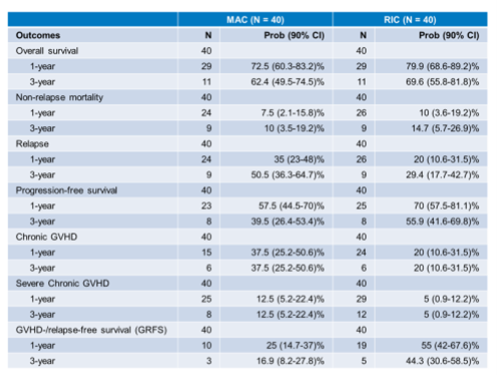
Figure 1: Overall Survival in the 7/8 and <7/8 matched cohorts
Shaw BE, et al., Journal of Clinical Oncology 1-year outcomes (3-year outcomes abstract pending publication in Bone Marrow Transplantation)
