According to research published with support from the CIBMTR® (Center for International Blood and Marrow Transplant Research®), the multicenter phase II trial Abatacept 2 (ABA2) shows reductions in acute graft-versus-host disease (GVHD) in both matched unrelated and mismatched unrelated donor hematopoietic stem cell transplantation (MUD and MMUD HCT) when abatacept is added to standard of care treatment. Effects were substantial in MMUD, where it was associated with a marked decrease in severe acute GVHD and transplant-related mortality (TRM), as well as improved severe acute GVHD-free survival (SGFS), relapse-free survival (RFS) and overall survival (OS). Results support abatacept as another promising tool to expand HCT access to those without a fully matched donor, which is more common for racially and ethnically diverse patients.
Download a PDF of the full study summary with journal citation here:
In the United States, Blacks and Hispanics are less likely than Whites to survive acute leukemias and other serious hematologic malignancies. Allogeneic HCT plays an important role in the treatment of high-risk hematologic malignancies and one factor likely contributing to this disparity is the limited availability of 8/8 HLA-matched unrelated donors for these patients. Although most racially and ethnically diverse patients have access to 7/8 MUD HCT, mismatching is often associated with increased risk for acute GVHD and reduced OS. This makes GVHD prevention tools like abatacept critical to the expansion of HCT access and the reduction of racial and ethnic disparities in HCT.
In January 2022, the U.S. Food and Drug Administration (FDA) approved abatacept for GVHD prevention in HCT. Researchers performed a post hoc analysis (published in Blood Advances) of the ABA2 trial data (used for FDA approval alongside real-world data presented in December 2021) to compare outcomes in patients receiving MMUD HCT with standard of care calcineurin inhibitor (CNI)/methotrexate (MTX) plus abatacept to just the standard of care treatment without abatacept. Outcomes show abatacept as a promising new drug for minimizing the historical risks associated with MMUD HCT.
ABA2(NCT01743131) compared CNI/MTX as the standard of care GVHD prevention strategy to CNI/MTX plus abatacept (trade name Orencia) in unrelated donor HCT. Patients with MMUD (7/8) were assigned to a single arm of the study receiving the standard of care plus abatacept and then compared with a pre-specified CIBMTR® registry cohort who received the only the standard of care.
Based on the substantial benefits seen in ABA2 for MMUD HCT, the study team hypothesized that abatacept could mitigate the risks associated with HLA mismatching. Therefore, the study team performed a secondary analysis of trial data to rigorously assess this, comparing outcomes in patients with MMUD receiving standard of care plus abatacept to patients with MUD receiving standard of care alone.
The median patient age was 40 years old (6.6 – 76.6 years). The groups differed only by distribution across conditioning regimens, with a more significant proportion (18.6% vs. 2.9%) of MMUD/abatacept patients receiving busulfan and fludarabine, and a smaller proportion of MMUD/abatacept patients receiving total body irradiation and cyclophosphamide (25.6% vs 37.7%). In addition, there were 30.2% non-White MMUD/abatacept patients vs. 11.6% non-White MUD/standard of care patients.
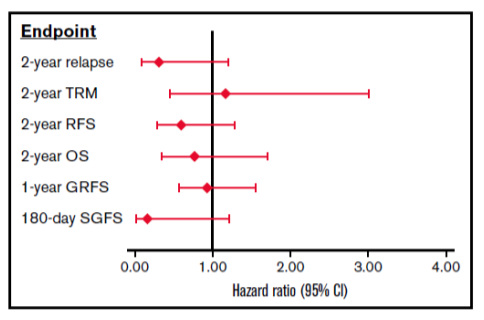
There were no differences in neutrophil or platelet engraftment, severe chronic GVHD, CMV or EBV viral infections, TRM, relapse, or RFS (see Figure 1B), GVHD free/relapse-free survival (GRFS) and OS between the groups. Severe acute GVHD (grades 3-4) (see Figure 1A) and SGFS were significantly lower in the MMUD/abatacept group compared to MUD/standard of care group. Multivariate analysis controlling for differences in patient age, performance score, disease stage, graft type and conditioning regimen between the groups did not impact these results (see Figure 2). The combined results in the following figures suggest that the addition of abatacept to standard CNI/MTX mitigates the disadvantages of mismatching by greatly reducing the risks of severe acute GVHD and non-relapse mortality without increasing the risk of relapse.
Options for ethnically diverse patients may considerably expand if results continue to show favorable outcomes in mismatched unrelated donor transplantation. The National Marrow Donor Program® (NMDP)/Be The Match® alongside the CIBMTR is committed to expanding access to all patients in need of HCT. Our research programs are developing and evaluating novel treatment strategies including abatacept, that allow for safe and effective use of mismatched unrelated donors expanding access to more patients in need.
Figure 1
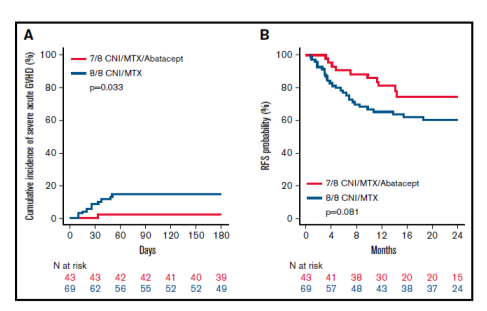
Figure 2