Patients treated with abatacept in combination with the current standard of care (SOC) for acute graft-versus-host disease (aGVHD) prophylaxis after mismatched unrelated donor (7/8 MMUD) allogeneic hematopoietic stem cell transplantation (HCT) had significantly better survival outcomes. These results presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition show abatacept as another promising GVHD prevention strategy that could expand the use of MMUD HCT.
Download a PDF of the full study summary with journal citation here:
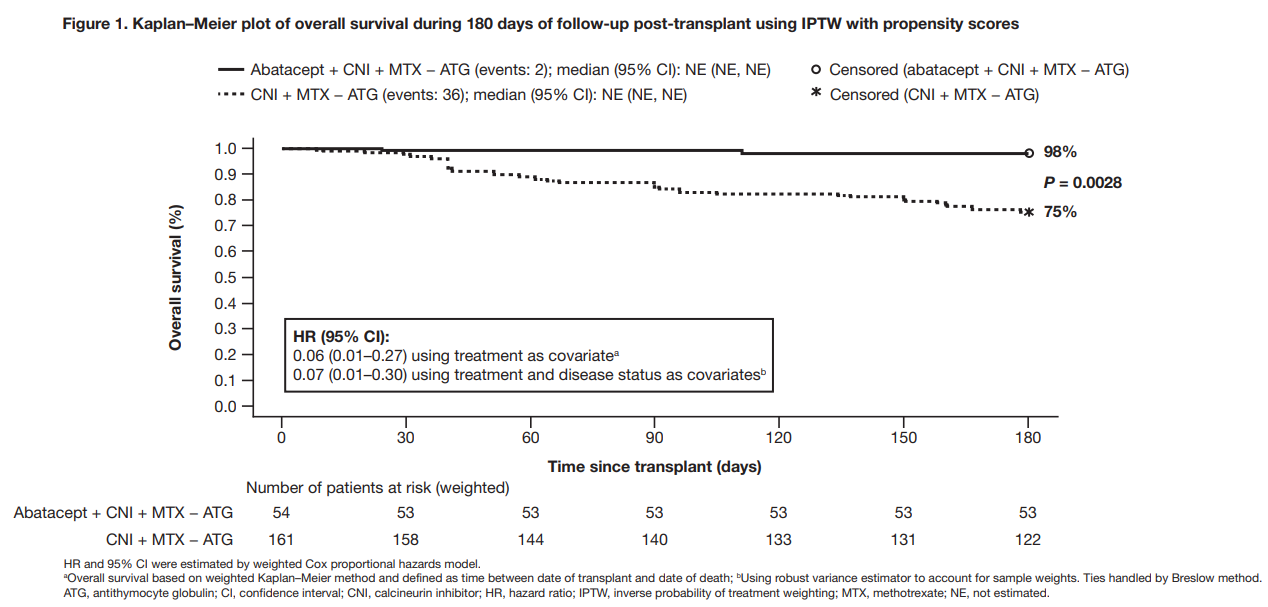
aGVHD is the most common cause of early non-relapse mortality following allogeneic HCT. Abatacept, a drug used to treat rheumatoid arthritis, blocks a signal that activates donor immune cells limiting their ability to cause aGVHD. A previous study reported 73.6% overall survival (OS) at 2 years in recipients of a 7/8 HLA-matched unrelated donor (7/8 MMUD) HCT following treatment with abatacept + SOC for aGVHD prevention (calcineurin inhibitor [CNI] + methotrexate [MTX] without [−] antithymocyte globulin [ATG]), compared with 45.3% in a SOC treatment cohort of matched controls from the Center for International Blood and Marrow Transplant Research (CIBMTR). The aim of this real-world analysis was to further evaluate the impact of abatacept on survival of 7/8 MMUD HCT recipients from the CIBMTR database of all allogeneic HCTs performed in the U.S. in recent years.
Patients 6 years of age or older with leukemia, lymphoma, or myelodysplastic syndrome (MDS) whose first allogeneic HCT was with a 7/8 MMUD between 2011 and 2018 were included in this study. Patients were treated with SOC aGVHD prophylaxis (CNI + MTX − ATG) with or without abatacept. Exploratory analyses of OS were also evaluated in 7/8 MMUD HCT recipients treated with abatacept + CNI + MTX (without ATG) versus CNI + MTX + ATG, and versus those treated with post-transplant cyclophosphamide-based GVHD prophylaxis.
OS rates at day 180 post-transplant were 98% for patients treated with abatacept + SOC compared to 75% for those treated with SOC only. The exploratory analysis showed that patients who were treated with abatacept + SOC did better than those treated with SOC alone (either CNI + MTX + ATG or post-transplant cyclophosphamide). Patients treated with abatacept had significantly better OS at day 180 compared with patients treated with only SOC.
Researchers concluded that abatacept has the potential to be another promising GVHD prevention tool that could expand use of MMUD transplants. Now the only FDA approved drug for GVHD prevention as of January 21, 2022, practice change could be warranted.
Overall Survival