In this Phase II study, researchers aimed to evaluate abatacept's efficacy in treating steroid-refractory chronic graft-versus-host disease (cGVHD) in patients after allogeneic hematopoietic cell transplantation (alloHCT). Results showed patients had promising overall response rates, with significant improvements in lung, liver, gastrointestinal tract, and mouth symptoms.
Download a PDF of the study highlights and citation.
Notably, inclusion of abatacept in treatment facilitated a lasting reduction in corticosteroid (prednisone) use, which is critical for reducing the negative effects of long-term steroid usage. The study also reported a favorable safety profile for abatacept with minimal severe adverse events.
Findings suggest abatacept could be a breakthrough therapeutic approach for patients who do not adequately respond to existing treatments, potentially improving outcomes and quality of life in the challenging landscape of post-HCT complications.
Background
The effectiveness of current treatments, including systemic corticosteroids and FDA-approved drugs, is limited, with incomplete responses and potential adverse effects. Treating cGVHD is an ongoing challenge that requires robust treatment options. Abatacept, an immunomodulator used for rheumatoid arthritis, has been studied for its potential to combat cGVHD by blocking T cell activation.
Initial Phase I clinical trials have indicated the safety and efficacy of this treatment in steroid-resistant cGVHD patients. This Phase II study aimed to evaluate abatacept's overall clinical response rate in patients with steroid-refractory cGVHD. The drug's unique mechanism, which hinders acute and chronic GVHD by competing with CD28 on T cells to bind to CD80/86 on antigen-presenting cells, has generated considerable interest in offering a treatment approach for cGVHD patients unresponsive to existing therapies.
Study Details
This study involved 39 patients with progressive steroid-refractory cGVHD despite current immunosuppressive treatments. All patients had undergone alloHCT with bone marrow (10%) or peripheral blood stem cell sources (90%) and received myeloablative (62%) or reduced-intensity conditioning (36%). Most participants were female, with an ECOG performance status of 1-2.
The primary diseases leading to transplantation were acute myeloid leukemia (46%) and myelodysplastic syndrome (20%). Others included acute lymphoblastic leukemia, chronic myeloid leukemia, non-Hodgkin’s lymphoma, myeloproliferative neoplasms, and myelofibrosis.
Results
In patients that received 6 doses of abatacept, 58% (21 out of 36 patients) achieved an overall partial response. Before taking abatacept, patients had received a median of 3 prior lines of systemic therapy for cGVHD and other immunosuppressive agents such as corticosteroids, calcineurin inhibitors, mycophenolate mofetil, ruxolitinib, and aldesleukin.
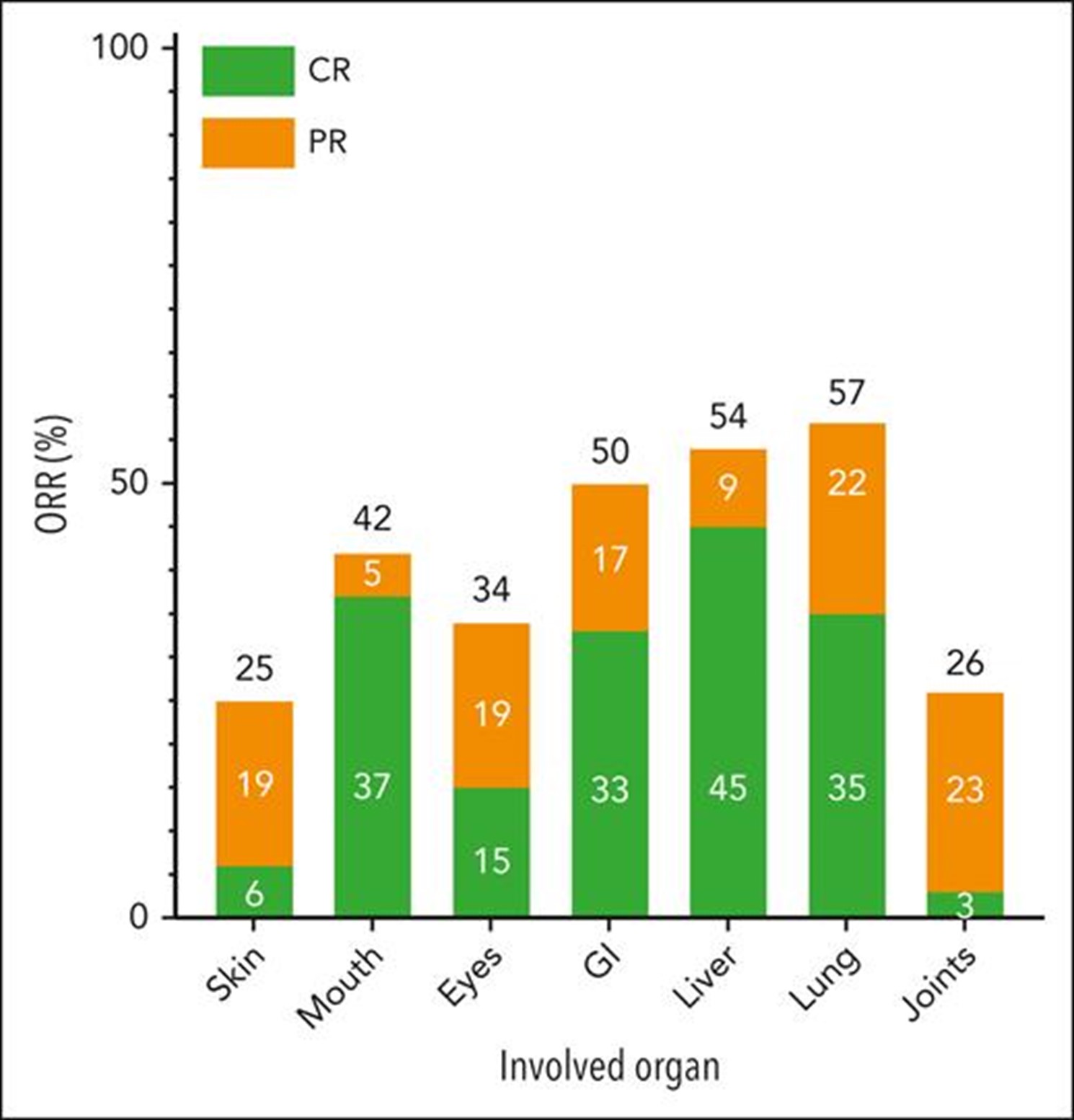
The organs showing the most significant improvement were the lung (57%), liver (54%), gastrointestinal tract (50%), and mouth (42%), as shown in the Figure below. However, cGVHD progression was noted in 33% of patients. No specific factors were associated with the overall response to abatacept.
Patients who responded to the treatment experienced a durable reduction in prednisone dose compared to non-responders. The 3-year overall survival rate among survivors was 72%. The most common adverse events potentially related to abatacept were neutropenia, fatigue, headache, and upper respiratory infection. Very few severe adverse events were reported, though four patients died during the study.
Immune correlative studies revealed decreased PD-1 expression on circulating CD4+ T cells post-treatment. Although significant differences in plasma concentrations of certain cytokines were observed pre- and post-treatment, no statistical difference was found in baseline cytokine levels between responders and non-responders.
Key Takeaways
This study reveals the potential of abatacept to treat steroid-refractory cGVHD for alloHCT recipients, with a 58% complete or partial response rate and significant improvements in patients' conditions.
Abatacept's immunomodulatory effects and the possibility of reducing corticosteroid use contribute to its promise, and its favorable safety profile further emphasizes its potential as a novel treatment approach. However, the absence of complete responses emphasizes the ongoing challenge in disease management and the need for continued research, more extensive trials, and exploration of combination therapies to fully harness its therapeutic benefits to optimize patient outcomes.
Koshy AG, et al., Published in Blood
