Researchers conducted a novel phase I clinical trial to evaluate the safety and feasibility of allogeneic, anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed/refractory multiple myeloma. Early data showed that 56% of patients responded, with 35% having a good partial response. This study suggests allogeneic CAR T-cell therapy may be a safe, viable treatment for relapsed and refractory multiple myeloma.
Background
Multiple myeloma is an incurable cancer, though treatment advancements have significantly progressed. New medications combined with autologous hematopoietic cell transplantation (HCT) and autologous CAR T-cell therapy have contributed to longer patient survival. However, relapse hinders survivorship due to diminishing effectiveness of each subsequent treatment.
CAR-T cell therapy has traditionally used autologous products, but this approach requires weeks to generate the product, and, in some instances, is not feasible due to the severity of patient disease. This study evaluated the use of an allogeneic CAR T-cell therapy product, called ALLO-715, that targets BCMA on certain cells, which is a common marker used in treating multiple myeloma. This trial aimed to determine if ALLO-715, the first allogeneic BCMA-targeted cell therapy, is safe and feasible, exploring optimal dosing. Additionally, this study included a new strategy for lymphodepletion called ALLO-647, which is a way to prepare the body to accept the CAR T-cells.
Study Details
The study cohort includes adult patients with relapsed/refractory multiple myeloma that have received at least 3 previous lines of therapy. Patients with donor-specific antibodies to the ALLO-715 product were excluded.
Patients received ALLO-715 at 1 of 4 possible dose levels. Before receiving ALLO-715, they were given ALLO-647 (anti-CD52 antibody) in combination with fludarabine and/or cyclophosphamide. Patients were separated into parts A, B, and C. Part A is still enrolling, but this publication includes interim results from 48 patients treated from September 10, 2019 – October 14, 2021. The median time from enrollment to the start of lymphodepletion was 5 days.
Results
The results from the interim cohort showed that five patients didn’t proceed to lymphodepletion because of progressive disease, death, or acute events. The remaining patients (N=43) received ALLO-715, which was made from 3 healthy donors and stored for off-the-shelf use. Results showed 33 patients discontinued treatment: 24 due to progressive disease, 7 related to death, and 2 withdrew consent.
Adverse events were expected related to lymphodepletion. All patients reported at least 1 adverse event, including 88% reporting events grade 3 or higher. After ALLO-715, 10 patients died from disease progression or grade 5 infections. Cytokine release syndrome (CRS) occurred in 56% of patients with only 1 grade 3 or higher case. Events of potential neurotoxicity occurred in 14% of patients, and all cases were grade 1 or 2, not requiring treatment with steroids. No cases of graft-versus-host disease (GVHD) were reported.
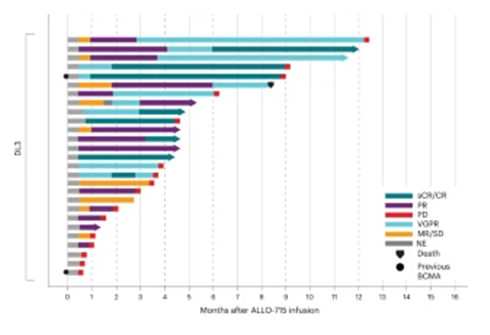
More than half of the patient cohort responded to treatment (56%), with 35% having a very good partial response (VGPR) or better within a median follow-up of 10.2 months, as shown in the figure below. The level 3 dosage was selected to move forward with 17 additional patients for different lymphodepletion strategies, and 71% achieved a partial response or better. 25% of patients were in complete or stringent complete remission with a median response time of 16 days and a median response duration of 8.3 months.
Key Takeaways
The study showed early evidence of the feasibility and safety of ALLO-715 use for relapsed/refractory multiple myeloma patients, which don’t have many options for this incurable, aggressive disease. Allogeneic donor cells can be accessed quickly, reducing the wait time for patients needing effective treatment. The trial is still enrolling patients, but preliminary results show allogeneic CAR T-cell therapy may be a safe and viable option for multiple myeloma patients.
Figure
Mailankody S, et al., Published in Nature Medicine
