According to research presented at the 2022 Tandem Meetings of the American Society of Transplantation and Cellular Therapy (ASTCT) and the CIBMTR® (Center for International Blood and Marrow Transplant Research®), racially and ethnically diverse patients considering hematopoietic stem cell transplantation (HCT) are underrepresented in clinical trials conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). Data suggest major barriers not only for enrollment in clinical trials, but for ensuring underrepresented groups (URGs) are included in the overall pipeline for HCT consideration.
Disparities in health care among racial and ethnic groups are well established. Patients from URGs (groups other than non-Hispanic whites) considering HCT face many additional barriers to care. This study examined URGs’ participation in BMT CTN clinical trials, specifically 9 trials from 2014 to 2021. Results were compared to existing data on racial and ethnic group breakdowns, including: 1) the total United States population as taken from the 2020 census, 2) all potentially eligible HCT recipients in the CIBMTR population in the United States at the time the trial was open for enrollment, and 3) all potentially eligible HCT recipients at centers participating in each trial from CIBMTR at the time of enrollment.
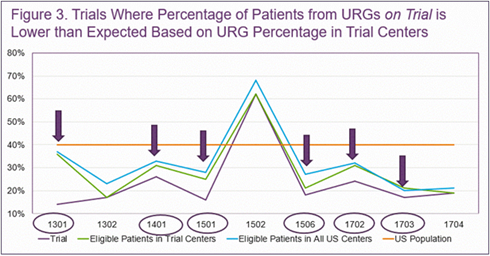
Results showed that 8 of the 9 trials had a lower proportion of participants from URGs than the general United States population. All but 1 of the 9 trials had a lower proportion of participants from URGs than potentially eligible HCT recipients in the
United States when the trial was enrolling, and 6 of the 9 trials had a lower proportion than those potentially eligible at participating trial centers (see Figure 3 shown below). Additionally, the researchers compared the proportion of patients from
URGs at trial centers compared to all United States centers and found that 3 of the 9 trial centers had a lower proportion of patients from URGs.
These results show a clear need for improved, targeted enrollment into clinical trials, as
well as into the overall pipeline for consideration for HCT. Patients are most likely to participate in a trial when asked by a trusted provider. The researchers stress the need for providers and institutions to take the time to develop trust with
their patients, and to be sure to ask all patients who may be eligible if they are interested in participating. Research suggests that less than 8% of patients with cancer participate in clinical trials, despite the fact that more than 50% will actually
participate when explicitly offered the option (Woodcock et al., 2021).
People from URGs face major barriers in accessing HCT,
with clinical trials showing the same issues. Ensuring that all eligible patients are considered for HCT through an initial consult with a regional transplant center physician can help alleviate barriers to HCT. Similarly, increased knowledge of available
clinical trials, as well as establishing a greater presence in communities and institutions where people from URGs receive treatment, is critical to improve access to this life-saving therapy and clinical trials.
Lower than Expected Proportion of URGs at Participating Trial Centers
Horowitz, et al., Tandem oral presentation abstract
