A research study presented at the 64th American Society of Hematology Annual Meeting and Exposition quantified the ongoing risk of hematopoietic cell transplantation (HCT) unrelated donors (URD) becoming infected with COVID-19 and the impact on collections. The number of donors testing positive for COVID-19 increased 2.4-fold in the first half of 2022 compared to the first half of 2021 (n=53 vs. 22).
Donors that tested positive caused transplant delays, including for patients who already started conditioning and needed to proceed with a different donor. Donor positivity delayed all patients that had not yet started conditioning. Most collection centers surveyed, both bone marrow and apheresis centers, reported they would not proceed with collection if a donor tested positive for COVID-19 if the recipient started conditioning. This emphasizes the need for transplant centers to have a viable backup plan if donors test positive.
Background
The global demand for HCT grafts from URD continues to grow despite the COVID-19 pandemic. The pandemic has challenged URD registries to adapt to the changing landscape of SARS-CoV-2 variants. While travel logistics have improved, the Omicron surge, particularly with the BA.5 subvariant, continued to place donors and patients at risk of developing COVID-19.
The National Marrow Donor Program® (NMDP)/Be The Match® and other registries mandated product cryopreservation at the beginning of the pandemic and reinstituted a mandate in January 2022 in response to the Omicron surge. NMDP/Be The Match removed the mandate in March 2022 but has recommended cryopreservation and a strong backup plan for patient safety. In this analysis, researchers sought to quantify the risk of donors becoming infected with COVID-19 from May 2021 to June 30, 2022.
Study Details
The study cohort reviewed NMDP/Be The Match donations from May 2021 to June 30, 2022, as well as surveys from Network apheresis centers (n=45) and bone marrow collection centers (n=32). The study team quantified the total number of donors testing positive for COVID-19 following medical clearance.
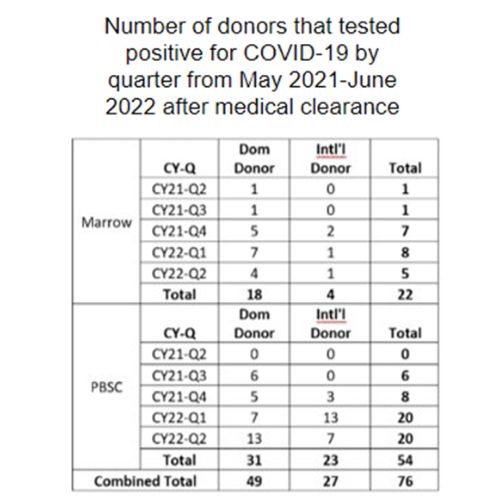
Results
The number of donors that tested positive for COVID-19 showed a 2.4-fold increase during the first half of 2022 compared to the first half of 2021 (n=53 vs. 22), as shown in Figure 1. Many donors tested positive before planned collection (n=76), including donors for adult recipients (n=57) and pediatric recipients (n=19). In addition, donors tested positive before planned fresh collection (n=33) and cryopreserved product transplant (n=43).
A subset of recipients of planned fresh products (n=20) had already started conditioning when the donor tested positive, which required rapid identification of alternative donors. While 9 of these 20 patients were eventually able to move forward with the same donor, 4 received a different but similar unrelated donor match, 1 received a different unrelated donor from another registry, 1 received a cord blood unit, and 5 cases are still open.
Donor positivity delayed transplants for all patients who did not start conditioning.
During the cryopreservation mandate periods of the pandemic (3/23/2020-8/10/2020 and 1/17/2022-3/14/2022), greater than 90% of NMDP/Be The Match products were cryopreserved compared to less than 10% before the mandate. The median requests for fresh compared to cryopreserved products throughout the pandemic was 58%, with rates varying by graft source.
Apheresis centers (65%) and bone marrow collection centers (50%) reported they would not proceed with collection if the donor tested positive for COVID-19 and the recipient had already started conditioning, including URDs that were asymptomatic. The survey found that in most cases of an asymptomatic URD testing positive for COVID-19, collection centers would not move forward with the collection.
Key Takeaways
The COVID-19 pandemic still poses logistical challenges to NMDP/Be The Match and other global URD registries. Transplant centers ordering fresh products following the initiation of conditioning should develop a plan among their teams. Transplant teams should also discuss the ongoing risk with patients undergoing conditioning before a URD product is available for infusion on the day of intended transplantation. Centers should have a viable backup donor plan or graft source if a donor tests positive for COVID-19 to prevent transplant delay.
Figure 1
Stefanski HE, et al., ASH Abstract
