Early interim analysis data from an ongoing study presented at the 2022 Tandem Meetings of the American Society of Transplantation and Cellular Therapy (ASTCT) and the CIBMTR® (Center for International Blood and Marrow Transplant Research®) show COVID-19 vaccination is less effective in those who have undergone cellular transplants and therapies in the last year, with more data needed to determine the ideal timing and number of boosters needed for this population. Vaccination continues to be important following hematopoietic stem cell transplantation (HCT) due to higher risk of COVID-19 infection in these vulnerable patients.
It is largely unknown how well the COVID-19 vaccines available in the United States work for people who have received HCT or CAR-T cell therapy, especially in the first year after treatment. Researchers think that patients’ immune systems’ response to the COVID-19 vaccine are likely not as strong as the general population, and early data seems to support that assumption. It is unknown when the best time to give the COVID-19 vaccine is and if there are markers that may predict how well the vaccine will perform for certain groups of patients.
In a joint ongoing study from the CIBMTR and the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), researchers are examining patients within a year of either HCT or CAR-T cellular therapy who got the COVID-19 vaccine in the United States. At the time of the abstract submission to the Tandem Meetings, 94 patients have joined the study. This study is still accepting new patients, with the goal of reaching over 500 participants by summer 2022. The median age of participants is 58 years (range, 11-77), and 57% (n=54) are male. The majority of patients had allogeneic HCT (61%; n=57), while 33% (n=31) had autologous HCT, and 6 patients (6%) had CAR-T cell therapy.
Blood samples were used to measure total antibody titers against the receptor-binding domain of the SARS-CoV-2 spike protein, assessing if there was an immune reaction triggered by the vaccine and if antibodies were successfully produced. Changes in antibody titers are shown in the figure below.
Early results show that none of the 6 CAR-T cell therapy patients in the study responded to the COVID-19 vaccine, meaning no antibodies were produced. Among all 30 patients receiving the vaccine, 77% of study patients developed antibodies after their second dose, and 57% of patients had more than a 4-fold increase (industry standard response to vaccination) in antibodies from their baseline level after their second dose (or the single shot for the one patient who got the Johnson & Johnson vaccine). For reference, 99% of non-immunocompromised patients would see such an increase in antibodies after their second shot under normal circumstances.
Results appear to be similar in those vaccinated less than 6 months versus at or after 6 months post treatment. Only 1 patient had a documented COVID-19 infection 137 days before getting the COVID-19 vaccine. Additionally, 5 patients got the COVID-19 vaccine before treatment with cellular therapy, and then received it again after treatment. Further work will examine T-cell responses more closely.
Based on these preliminary findings, there appears to be lower efficacy of the COVID-19 vaccine among patients treated with cellular therapy than the general population. More work needs to be done to determine optimal timing for giving the COVID-19 vaccination to this population, how many doses are necessary, and what the predictors of response to the vaccine may be after different cellular therapy treatments and for different patient groups. More data is expected in the near future. For now, COVID-19 vaccination within the first year of cellular therapy is still the standard to ensure protection for this vulnerable population.
Download a PDF of the full study summary here:
Change in Antibody Titers
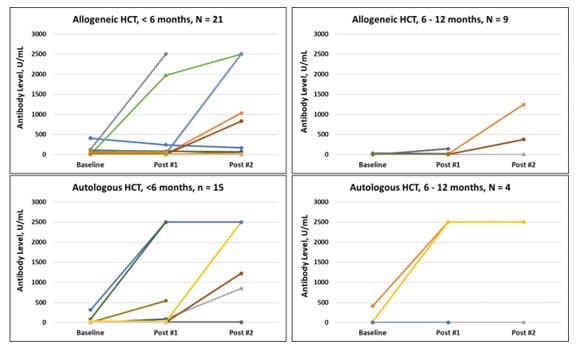
This figure shows the change in antibody titers from baseline through sample 2, collected 7-35 days following vaccine dose #2. The top panels are allogeneic HCT recipients, and the bottom panels are autologous HCT recipients based on time of vaccination after cell infusion [ <6 months (left), 6-12 months (right)]. Each line represents a single patient.
Riches ML, et al., Tandem poster presentation abstract
