This multi-center single-arm, phase-II, prospective clinical trial from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) aimed to assess the efficacy of reduced intensity haploidentical bone marrow transplant (haploBMT) using post-transplant cyclophosphamide (PTCy) for curative treatment of adults with severe sickle cell disease (SCD). The research is pivotal in exploring alternative curative therapies for SCD patients who lack a matched sibling donor (MSD) and are ineligible for myeloablative conditioning due to its toxicity.
Study Background
Sickle cell disease is a severe hereditary blood disorder characterized by the sickling of red blood cells, leading to pain, anemia, and organ damage. Allogeneic BMT from a MSD has shown promising curative potential with a high event-free survival (EFS) rate in children. However, the majority of SCD patients do not have an MSD, and adults face significant risks from the toxicity associated with full-intensity conditioning. This study, BMT CTN 1507, explores the viability of haploBMT with PTCy as a less toxic alternative, addressing the challenges of graft failure and graft-versus-host disease (GVHD).
Methods
The trial enrolled SCD patients aged 15-45 with severe disease manifestations, including prior stroke, recurrent acute chest syndrome, or chronic transfusion regimen. Participants underwent a preconditioning regimen followed by a conditioning regimen that included various chemotherapeutic agents and total body irradiation, with GVHD prophylaxis provided by PTCy, sirolimus, and mycophenolate mofetil. The primary goal was to estimate the 2-year EFS, with secondary objectives focusing on the impact on SCD manifestations and other transplant outcomes.
Results
Out of 54 participants enrolled, 42 proceeded to transplant. The majority were male and Black. The estimated 2-year EFS was 88%, with a 2-year overall survival (OS) post-transplant of 95%. Notably, all but one qualifying event for EFS occurred within the first 12 months. The cumulative incidence of acute GVHD by day 100 was 26.2% for grades II-IV and only 4.8% for grades III-IV. A significant portion of participants experienced re-admission post-BMT, primarily due to infections ; however, this trial was conducted during the COVID-19 pandemic. The outcomes demonstrate that haploBMT with PTCy is a viable and relatively safe option for adults with severe SCD, showing durable donor engraftment and low mortality comparable to MSD myeloablative BMT.
Key Takeaways
The study underscores the potential of haploBMT with PTCy as a curative treatment for adults with severe SCD, expanding the donor pool and offering a less toxic alternative to traditional myeloablative conditioning. The findings have significant implications for the industry, suggesting a new direction for treating SCD in adults with severe end-organ toxicity. Furthermore, the results hold promise for expanding to other donor types to improve accessibility and outcomes. The study advocates for increased awareness among health care providers and patients about this therapeutic option, highlighting the need for further research and funding to support access to this potentially life-saving treatment.
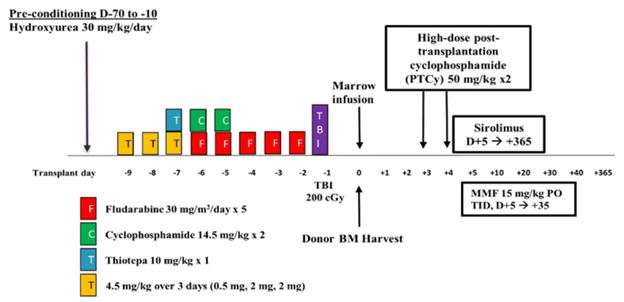
Figure
This figure displays the common conditioning regimen for haploBMT used in this study. Abbreviations include: MMF (mycophenolate mofetil), PO (by mouth), TID (three times daily), and TBI (total body irradiation).
Kassim AA, et al., ASH abstract published in Blood
