This trial aimed to evaluate the effectiveness of ibrutinib as a first-line treatment for newly diagnosed moderate or severe chronic graft-versus-host disease (cGVHD) in patients who had not received prior systemic treatment. The trial is the first of its kind, utilizing objective response criteria and employing a prospective, randomized, double-blind, placebo-controlled phase III design. Although the study's results did not demonstrate a statistically significant difference between the ibrutinib-prednisone and placebo-prednisone groups, duration of response, and overall survival (OS), it highlighted the ongoing need for research and more effective treatments for cGVHD.
Background
cGVHD is a significant cause of late non-relapse mortality and a barrier to successful allogeneic hematopoietic cell transplant (alloHCT). It affects up to 70% of alloHCT patients, with around 30-40% requiring systemic treatment. Corticosteroids are commonly used as the first-line treatment for cGVHD, but their long-term use has drawbacks, and there are no approved alternative therapies for previously untreated cGVHD. This study focuses on ibrutinib, a drug approved in the U.S. for adults with cGVHD who haven't improved after other systemic treatments.
Study Details
This study aimed to evaluate the effectiveness of ibrutinib as a first-line treatment option for newly diagnosed moderate or severe cGVHD in patients aged 12 years and older. Patients who required systemic corticosteroid therapy and had not received prior systemic treatment for cGVHD were randomly assigned in a 1:1 ratio to receive either ibrutinib 420 mg once daily in combination with prednisone or placebo plus prednisone.
The primary outcome measure was the response rate at 48 weeks based on the 2014 National Institutes of Health Consensus Development Project Criteria. Secondary endpoints included event-free survival (EFS), duration of response, time to withdrawal of immunosuppressants, Improvement in Lee cGVHD Symptom Scale score, OS, and safety.
Results
In this study, patients were enrolled into 2 arms receiving ibrutinib-prednisone treatment (n=95) or placebo-prednisone treatment (n=98). At the 48-week mark, there was no significant difference in the response rates (41% for the ibrutinib-prednisone group and 37% for the placebo-prednisone group). The median duration of response was 19 months and 10 months, respectively, at 33 months of follow-up. The median EFS was 15 months and 8 months, respectively, with a hazard ratio of 0.76 (indicating a trend towards better outcomes in the ibrutinib-prednisone group, but not statistically significant).
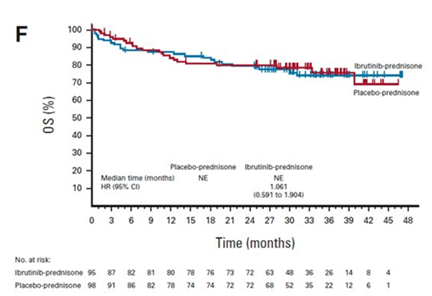
There was a 43% improvement in the overall Lee cGVHD Symptom Scale for the ibrutinib-prednisone group compared to 31% for the placebo-prednisone group (not statistically significant). The median OS was not reached in either group, as shown in the figure below. Grade 3 or higher adverse events occurred in 49% of patients in the ibrutinib-prednisone group and 47% in the placebo-prednisone group. Notably, more patients treated with ibrutinib-prednisone could withdraw from all immunosuppressants, and this difference was statistically significant.
Key Takeaways
The study highlights the ongoing unmet need for the treatment of cGVHD and emphasizes the importance of further research in this area. Despite the lack of positive outcomes, the field is actively working towards addressing the challenges associated with cGVHD across both treatment options and prevention strategies. The recent phase III clinical trial examining ibrutinib as a therapy for previously untreated cGVHD served as a valuable model for future investigations in this field. The study also provided helpful insights into the design of future clinical trials for testing cGVHD therapies and emphasized the importance of continued research in addressing the challenges of cGVHD treatment and prevention.
Figure
Miklos DB, et al., Published in Journal of Clinical Oncology
