Research presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition examined the concordance of patient data, specifically vital status and cause-specific mortality after first hematopoietic cell transplantation (HCT). Data were compared between the CIBMTR® (Center for International Blood and Marrow Transplant Research®) and the California Cancer Registry (CCR) databases.
Results showed the consistency of vital status between the data sets was high at 94%; however, differences between the reported data may be due to the length of follow-up and incomplete patient information. This study provides support for combining databases and future research collaborations that can expand our understanding of HCT outcomes.
Background
The CIBMTR is a fundamental resource for studying outcomes after transplant, but collaboration with other data registries could be valuable and enhance analyses. CIBMTR data regarding patient mortality are assumed to be under-reported because of the reliance on patient follow-up with their transplant center. CCR has mortality data for California residents; therefore, the combination of the two datasets allows for an improved understanding of outcomes after HCT.
Study Details
The cohort included 18,450 patients who underwent their first transplant for a blood cancer diagnosed in 1991 through 2016 with patient follow-up until 2018. There were 8,232 allogeneic (alloHCT) patients (45%) and 10,218 autologous (autoHCT) patients (55%). The median follow-up among patients alive at the end of follow-up was 4.4 years (range, 0.005-27.4) in the CIBMTR and 5.1 years (range, 0.01-27.5) in the CCR data.
Researchers compared information from the CIBMTR and CCR data on the last known vital status (dates within 90 days of each other were considered concordant) and primary cause of death (using ICD codes for CCR data and standard CIBMTR categories for CIBMTR data). For patients who were deceased, they compared whether the cause was cancer or non-cancer in each data set.
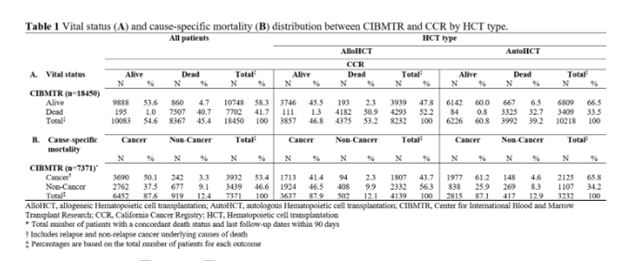
Results
The study found a 94% concordance of vital status between the two datasets, as shown in Table 1 below. There were 1,055 patients noted to be alive in one dataset but deceased in another, and 826 of those patients had a mortality date after the last known alive data in the other source.
This finding suggests differences in the length of follow-up reporting. CCR had a longer follow-up time and more complete ascertainment of vital status, while CIBMTR had more specific and relevant clinical information about cause of death, such as graft-versus-host disease (GVHD), infection, relapse or non-relapse.
This study showed that CCR reported the cause of death as cancer more frequently in deceased patients noted in both sources. The CIBMTR reported clinical details in the congruent list of deceased patients that CCR did not include, such as relapse mortality and GVHD as a possible non-cancer causes of death along with other reasons.
Key Takeaways
While the information within the CIBMTR database is incredibly important for studying HCT patient outcomes, collaboration with other organizations can provide a more well-rounded view of the data and aid in answering specific research questions.
Different databases can have different strengths, which can provide a more complete understanding of HCT outcomes and overall patient status. This study using CCR data is an example of this, but other applications and collaborations should be considered in the future to advance HCT research.
Table 1
Valcarcel B, et al., ASH poster presentation abstract
