This observational research provides a detailed examination of Medicaid's coverage for hematopoietic cell transplant (HCT) and cell therapy across all 50 states in the U.S., aiming to pinpoint areas needing intervention. The primary findings revealed marked gaps and variability in Medicaid coverage from state to state, suggesting significant challenges that could prevent patients from accessing life-saving treatments.
Download a PDF of the study highlights and citation.
Background
In 2021, 27.2 million people in the U.S. lacked health insurance, with 35.7% of those insured relying on public health coverage, predominantly through Medicaid. Concerningly, discernible disparities have emerged, particularly in HCT outcomes and utilization rates for patients diagnosed with various blood cancers and disorders. These disparities are more pronounced in racially and ethnically diverse groups.
To counter these inequities and barriers to care, NMDPSM and the American Society for Transplantation and Cellular Therapy (ASTCT) launched the ACCESS Initiative, jointly targeting challenges related to awareness, poverty, and racial/ethnic inequity. Given Medicaid's significant role in insuring a diverse and economically disadvantaged demographic, comprehending its coverage dynamics for transplant, cell therapy and donor costs is vital.
Study Details
The NMDP-funded Medicaid scan, executed by Manatt Health, meticulously analyzed the coverage and reimbursement strategies for HCT and chimeric antigen receptor T-cell (CAR-T) therapies. This encompassed an evaluation of the standard indications for both autologous and allogeneic HCT as endorsed by the ASTCT. Additionally, the research assessed coverage for notable CAR-T therapies and delved into policies concerning travel, lodging, meals, caregiver provisions and allogeneic donor expenses.
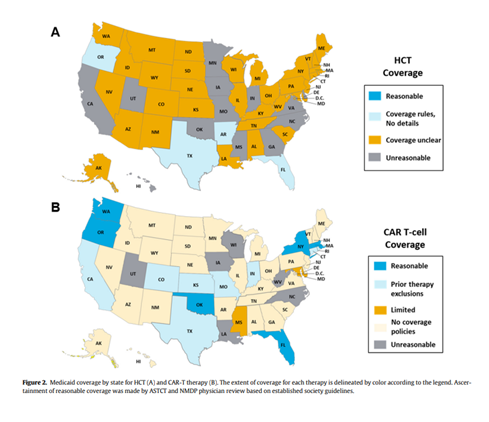
Results
Regarding HCT indications, no state fully conformed to the current ASTCT and NMDP guidelines. A concerning 10 states did not offer coverage for all of the studied disease indications, with many other states imposing unclear, unreasonable eligibility criteria. Other states set limitations based on age, mandated clinical trial enrollment, or presented idence-unsupported restrictions.
As for CAR-T indications, while most coverage was consistent with FDA labels, 14 states incorporated inappropriate restrictions. Several states did not have specific Medicaid policies for certain disease indications.
In terms of travel and lodging, all states promised transportation coverage, but the specifics and extent varied. A notable 7 states did not house any transplant centers within their borders. Moreover, policies regarding lodging and meals showed considerable variance, with some states implementing stringent conditions.
Caregiver coverage was primarily extended to Medicaid beneficiaries under 18 or when medically required for adults, with particular restrictions in place. Only a handful of states offered compensation for caregivers' lost earnings during travel.
There were also notable differences in how states managed direct payments and reimbursements. In many scenarios, beneficiaries had to shoulder upfront costs for services. When considering donor testing and procurement costs, states adopted diverse payment strategies for donor-related expenses, and a scant few considered costs associated with testing multiple donors.
Key Takeaways
This research underscores the profound inconsistencies and gaps that exist in Medicaid coverage across different states, illuminating barriers that could deny beneficiaries access to life-saving treatments. By bringing these disparities to light, the study sets the stage for addressing the evident shortfalls in Medicaid's support for HCT and CAR T-cell recipients.
As a proactive measure, subcommittees within the ACCESS Poverty Committee are working to address the identified challenges. The overarching ambition is not only to heighten awareness of these coverage voids but also to champion policy alterations and foster a collective commitment to ensuring all those in need can access these crucial treatments.
Auletta JJ, et al., Published in Transplantation and Cellular Therapy
